Chapter 7
- 7.1 Introduction
- 7.2 Objectives of Mine Drainage Treatment
- 7.3 Mine Drainage Treatment
- 7.4 Mine Drainage Collection and Management
- 7.5 Mine Drainage Treatment Technologies
- 7.5.1.1 Active Treatment Technologies – Neutralization/Hydrolysis
- 7.5.1.2 Active Treatment – Metals Removal
- 7.5.1.3 Active Treatment – Chemical Precipitation for Sulphate Removal
- 7.5.1.4 Active Treatment – Membrane Treatment
- 7.5.1.5 Active Treatment – Ion Exchange
- 7.5.1.6 Active Treatment – Biological Sulphate Removal
- 7.5.1.7 Specialized Treatment – Sulphide Precipitation
- 7.6 Treatment Residues and Wastes
- 7.7 Recovery of Useful Products
- 7.8 Treatment in the Context of Mine Closure and Post Closure
- 7.9 Evaluation and Selection of Drainage Treatment Technologies
- 7.10 References
- List of Tables
- List of Figures
7.0 Drainage Treatment
7.1 Introduction
- Objectives of and approach to mine drainage treatment
- Mine drainage collection
- Treatment technologies including:
- Active treatment
- Passive treatment
- Active/passive hybrids
- In situ treatment
- Treatment residues and waste
- Recovery of useful byproducts
- Drainage treatment during mine closure and post closure
- Selection of appropriate treatment technology
The objectives and approach to treatment of the different mine water types depend on the category of mine water and the degree of treatment required.
The consideration of drainage treatment technologies covers the range of applications to the following:
- Different commodities, including coal, diamond, iron, gold, uranium, and precious and base metals
- Different phases of mining, including exploration, feasibility (assessment and design), construction, operation, decommissioning, and post closure
7.2 Objectives of Mine Drainage Treatment
The objectives of mine drainage treatment are varied and may include one or more of the following:
- Recovery and reuse of mine water within the mining operations for processing of ores and minerals, conveyance of materials, and operational use (e.g., dust suppression, mine cooling, irrigation of rehabilitated land). Most mining operations include the management of water on the mine site and manage associated water infrastructure. The mine water balance requires management of different demands for water volume and water quality. Mine drainage treatment, in this case, is aimed at modifying the water quality so that the treated effluent is fit for the intended use on the mine complex or site.
- Protection of human health in situations where people may come in contact with the impacted mine water through indirect or direct use of mine water drainage.
- Environmental protection, specifically related to mining water impacts on surface water and groundwater resources. Mine drainage may act as the transport medium for a range of pollutants, which may impact on-site and off-site water resources. Water treatment would remove the pollutants contained in mine drainage to prevent or mitigate environmental impacts.
- Useful and potentially saleable products may be recovered from mine drainage. It is unlikely that byproducts recovery would be a sole driver to the installation of a water treatment facility. However, when commodity prices are high, the recovery of saleable products will improve the financial viability of mine drainage treatment projects.
- Regulatory requirements may stipulate a mine water discharge quality or associated discharge pollutant loads. Any discharge of mine drainage to a public stream or aquifer must be approved by the relevant regulatory authorities. Discharge quality standards may not be set for many developing mining countries, but internationally acceptable environmental quality standards may still apply as stipulated by project financiers and company corporate policies.
- Mine water is a valuable resource and much of the world is facing water stress. The beneficial use of mine water to satisfy the needs of a variety of mining and nonmining water users can be a key driver supporting the installation of mine drainage treatment facilities. There is an increasing number of mine drainage treatment projects aimed at supplying treated mine water to neighbouring communities and industries around mines.
- Sustainability of mining will require the mitigation, management, and control of mining impacts on the environment. In many cases, the mining impacts on water resources are long term and persist in the post-closure situation. Mine drainage treatment may be a component of overall mine water management to support a mining operation over the mine’s entire life and enhances post-closure and sustainable use of the mine property long after the ore deposit is depleted.
7.3 Mine Drainage Treatment
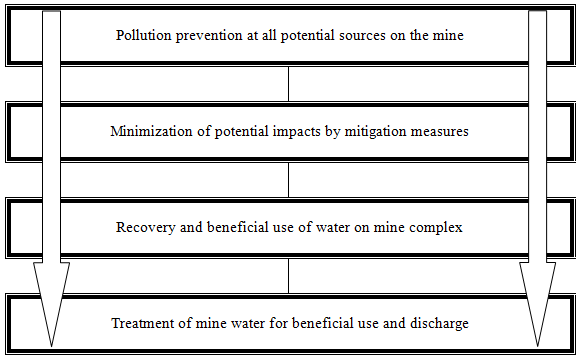
The approach to mine drainage treatment is based on an understanding of the integrated mine water system and circuits and the specific objective (or objectives) to be achieved. A generic mine water system diagram is shown in Figure 7-1 to demonstrate the point that treatment may be introduced at several different points or locations on a mining project and to illustrate different purposes and objectives.

The generic location for a mine drainage treatment facility includes the following:
- A selected mine water stream originating from a process or facility discharging high concentrations and loads of pollutants
- A water stream dedicated to some mining-related water use, which may require a specific water quality
- A return water stream to render the recycled water fit for use in the mining or minerals processing operation
- A point or diffuse discharge stream to a natural watercourse or aquifer
Mine drainage treatment projects are executed within the overall hierarchy of mine water management, which generically includes the following steps:
This approach adopted for mine drainage treatment will be influenced by a number of considerations related to the following:
- Before selecting the treatment process, a clear statement and understanding of the objectives of treatment should be prepared. Mine drainage treatment must always be evaluated and implemented within the context of the integrated mine water system. Treatment will have an impact on the flow and quality profile in the water system; therefore, the sized treatment system is selected based on mine water flow, water quality, cost, and ultimately water uses.
- Characterization of the mine drainage in terms of flow and key properties of ARD, NMD, or SD should include careful consideration of temporal and seasonal changes. Flow data are especially important because this information is required to properly size any treatment system. Particular concern should be taken with the extreme precipitation and snowmelt events to ensure that the collection ponds and related piping and ditches are adequately sized. The key properties of mine drainage relate to acidity and alkalinity, sulphate content, salinity, metal content, microbiological quality, and the presence of specific compounds associated with specific mining operations, such as cyanide, ammonia, nitrate, arsenic, selenium, molybdenum, and radionuclides. There are also a number of properties of the mine-drainage constituents (e.g., hardness, sulphate, and silica) that may not be of regulatory or environmental concern in all jurisdictions, but that could affect the selection of the preferred water treatment technology.
- Different stages of mining and how the mine water system and water balance will change over the life of a mine. A mine drainage treatment facility must have the flexibility to deal with increasing and decreasing water flows, changing water qualities, and regulatory requirements. This may dictate phased implementation and modular design and construction of a treatment facility. Additionally, the post-closure phase may place specific constraints on the continued operation and maintenance of a treatment facility.
- Commodity-specific water aspects related to compounds present in the mine drainage (e.g., presence of radionuclides in the case of uranium mining). Some mining or processing operations may introduce extraneous chemicals and reagents into the water circuits. Reagents from one minerals processing plant (e.g., copper recovery) may be detrimental to another minerals processing plant (e.g., phosphate recovery).
- Practical mine site features, which will influence the construction, operation, and maintenance of a mine drainage treatment facility, including the following:
- Mine layout and topography
- Space
- Climate
- Sources of mine drainage feeding the treatment facility
- Location of treated water users
- Handling and disposal of treatment plant waste and residues, such as sludges and brines
7.4 Mine Drainage Collection and Management
The collection and conveyance systems and infrastructure pose engineering and operational challenges because of the variable flow rates and the corrosive or scaling nature of mine drainage. The considerations in the development of a mine drainage collection and conveyance system include the following:
- Properties of mine drainage, including corrosiveness, scale/precipitate forming potential, solids deposition, organic fouling, and plugging
- Dealing with variable mine drainage flows and qualities as dictated by climatic and seasonal changes and by the different stages of the life of the mine (The sizing of collection ponds and ditches is particularly critical where combined snow or precipitation events can combine to over top and cause failure of these facilities.)
- The size of the collection ponds and ditches may be defined by the regulatory requirements (i.e., to meet a 24-hour 100-year precipitation event)
- Site and route selection based on consideration of topography, geotechnical conditions, and climate
- Selection of appropriate materials of construction
- Engineering features, including pretreatment before conveyance, pumping installation, and piping systems
- Operational aspects related to access, regular cleaning, monitoring, typical failures, and risks
- Maintenance aspects, particularly ease of cleaning
Mine drainage collection and conveyance systems are critical components of any treatment project. Appropriate basis of design must be developed and integrated into the overall treatment project. Surge ponds may be a valuable feature in the case of highly variable mine drainage flows and pollutant loads. This will afford some protection against surcharging the treatment system. Examples exist of failed projects because of the neglect of the design and operation of the mine drainage collection infrastructure.
7.5 Mine Drainage Treatment Technologies
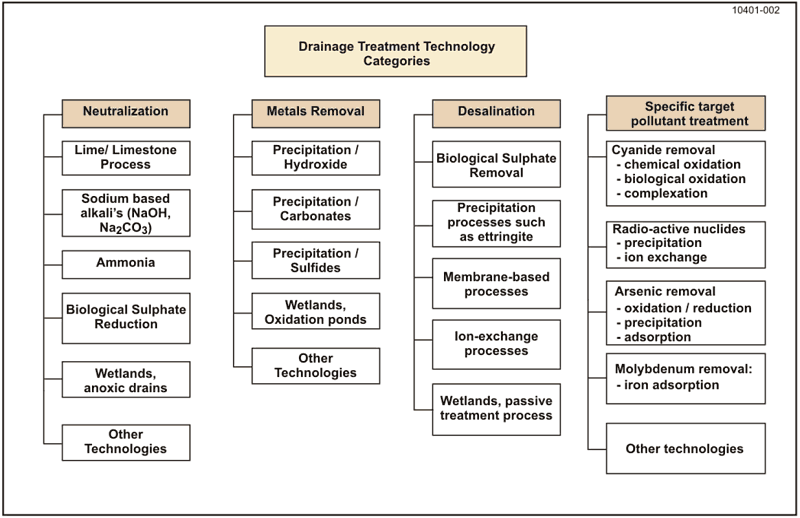
A wide spectrum of drainage treatment technologies has been developed, proven, and applied to many different applications. The generic range of mine drainage treatment technologies is reflected in Figure 7-2: Generic Range of Drainage Treatment Technologies. The description of the different drainage treatment technologies in this Section 7.5 will be framed in the context of current best practice of proven technologies.
Mine drainage treatment technologies can be broadly classified into active treatment, passive treatment, and in situ treatment as described in Table 7-1. The selection of the appropriate category of mine drainage for a specific application is influenced by the aspects summarized in Table 7-1.
Feature / Characteristic |
Active Treatment |
Passive Treatment |
In Situ Treatment |
| 1. Application to phase of mining | Most appropriate to exploration and operational phases because it requires active control and management. Closure and post-closure applications mainly associated with large flows. | Most attractive to the closure and post-closure phases, because it requires only intermittent supervision, maintenance, and monitoring of self-sustaining processes. | Appropriate to the exploration and operational phases because it requires ongoing operation and maintenance. |
| 2. Operational involvement | Active and ongoing plant operations and maintenance systems and personnel. | Constant operations not required, but regular maintenance essential. | Active and ongoing operational personnel required, but permanent presence on site not required. |
| 3. Operational inputs and materials | Requires chemicals, operations staff, maintenance staff, electrical power, continuous and/or regular monitoring. | Self sustaining processes, periodic maintenance, intermittent monitoring. May require replacement or supplement of materials at low frequency. | Requires chemicals, operations staff, intermittent field maintenance, electrical power and low frequency monitoring. |
| 4. Supply of power | Electrical and mechanical energy sources. | Natural energy sources of gravity flow, solar energy and bio-chemical energy. | Electrical and mechanical energy sources. |
| 5. Management and supervision requirements. | Ongoing management engagement, constant facility supervision. | Low level management engagement and low frequency intermittent supervision. | High frequency supervision, but no permanent site presence required. |
6. Range of application:
|
Application to all flow rates, especially high flow rates and any constituent of interest. | Mainly applied to low flow rates and acidity, metals, and sulphate removal. | Large spectrum of volume and flow applications, mainly to deal with acidity and metals removal. |
| 7. Treated water quality | Treatment process can be purpose built to deal with spectrum of treated water requirements. | Treated water quality poorer and more variable than other options. | Treated water quality lower and more variable than active treatment process. |
| 8. Waste sludge and brine production. | Waste sludge and brine are produced, depending on level of treatment, requiring disposal. | No brine production, but longer term liability to deal with accumulated pollutants in wetlands sludges. | Sludge and waste production accumulated in situ, may pose long term environmental liability. |
| 9. Capital investment cost | High capital investment and periodic capital replacement required. | Moderate capital investment with periodic reinvestment to replace depleted wetlands media. | Low capital investment typically to deal with a short term problem. |
| 10. Operating and maintenance cost | High operating and maintenance cost, with some potential for cost recovery by sale of product water, metals and byproducts. | Low operating cost. | Moderate operating costs, but chemical usage may be high due to process inefficiency. |
7.5.1 Active Treatment Technologies
Active treatment refers to technologies requiring ongoing human operations; maintenance, and monitoring based on external sources of energy (electrical power) using infrastructure and engineered systems
Active treatment technologies include neutralization, which often includes metal precipitation, metals removal, chemical precipitation, membrane processes, ion exchange, and biological sulphate removal. The key considerations in selecting an appropriate neutralization agent and integrated process configuration for a specific mine water treatment application include the following:
- Materials handling, including road/rail transport, bulk storage, make up, and dosing
- Classification of alkali material as a dangerous or hazardous material requiring special precautions in handling and personnel safety
- Availability and reliability of supply
- Efficiency as neutralizing agent and active ingredient/component of bulk material
- Process implications such as increasing propensity for scaling/coating/clogging of equipment /pipelines/instrumentation
- Infrastructure and equipment investment cost of alkali material handling, storage, make up, and dosing facilities
7.5.1.1 Active Treatment Technologies – Neutralization/Hydrolysis
Neutralization and hydrolysis are key aspects of ARD treatment and many different alkali materials and different process configurations are employed. A list of commonly applied alkali compounds and materials is in Table 7-2.
Alkali Compound/Material |
Alkali Requirements |
Neutralisation Efficiency |
Relative Cost |
| Limestone, CaCO3 | 1.00 | 30 - 50 | 10 – 15 |
| Hydrated lime, Ca (OH)2 | 0.74 | 90 | 60 – 100 |
| Un-hydrated (quick) lime, CaO | 0.56 | 90 | 80 – 240 |
| Soda ash, Na2 CO3 | 1.06 | 60 - 80 | 200 – 350 |
| Caustic soda, Na OH | 0.80 | 100 | 650 – 900 |
| Magna lime, MgO | 0.4 | 90 | Project specific |
| Fly ash | Material specific | - | Project specific |
| Kiln dust | Material specific | - | Project specific |
| Slag | Material specific | - | Project specific |
2Neutralization efficiency estimates the relative effectiveness of the chemical in neutralizing AMD acidity. For example, if 100 tons of acid was the amount of acid to be neutralized, then it can be estimated that 82 tons of hydrated lime would be needed to neutralize the acidity in the water (100(0.74)/0.90).
3Price of chemical depends on the quantity being delivered. Bulk delivery prices and small quantity delivery prices will differ. These prices are approximate and generally reflect the market in January 2009. Prices will vary significantly around the world and over time.
Selection of an alkali material depends on the following:
- Secondary impacts associated with the use of a specific alkali residual on treated mine water quality such as ammonia content (aquatic environmental, eco-toxicity impacts), and increased salinity
- Cost of alkali material
- Treatment objectives, specifically the removal of metals
Lime neutralization in a high density sludge (HDS) process configuration is the industry standard for impacted mine water neutralization for of the following reasons:
- Relative low cost of lime
- Efficient use of lime
- High density of waste sludge requiring a smaller site for disposal
- Scale control on treatment plant structures, pipelines, equipment, and instrumentation
- Good solids/water separation
- Robust process, able to treat variable flows and acidity/metals loadings
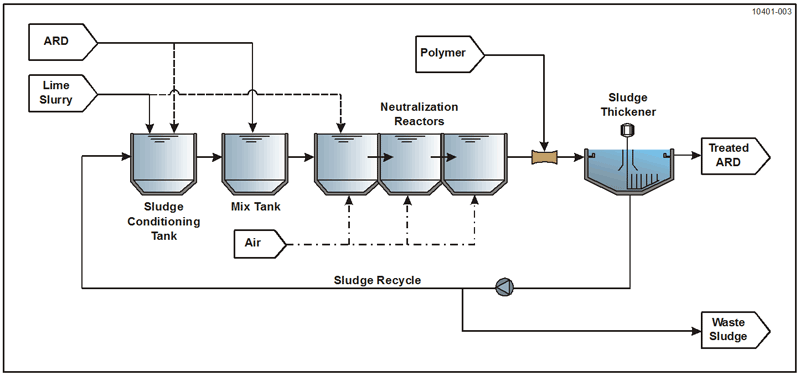
Lime neutralization/hydrolysis in an HDS process configuration is the most established and widely practiced ARD treatment technology. A number of variations and innovations to the original HDS treatment process concept have been developed and implemented. The basic HDS process configuration is shown in Table 7-3.
The key features of some of the commonly applied HDS process variations are shown in Figure 7-3.
Process Parameters |
Conventional HDS |
Cominco Process |
Geco Process |
Staged-neutralization |
Tetra (Doyon) Process |
| ARD feed point | Mix tank | Mix tank | Sludge conditioning tank | First stage | Sludge conditioning tank |
| Sludge recycle point | Sludge conditioning tank | Separate sludge/lime mix tank | Sludge conditioning tank | Upstream stages | Sludge conditioning tank and Separate sludge/lime mix tank |
| Lime slurry feed point | Sludge conditioning tank | Separate sludge/lime mix tank | Rapid mix tank | Downstream stages | Separate sludge/lime mix tank |
| Aeration, air injection | Neutralization reactor | Neutralization reactor | Neutralization reactor | Upstream stages | Neutralization reactor |
| Polymer addition point | Upstream of thickener | Upstream of thickener | Upstream of thickener | Upstream of thickener | Upstream of thickener |
| Solids separation device | Gravity thickener | Gravity thickener | Gravity thickener | Gravity thickener | Gravity thickener |
The selection of the most appropriate lime neutralization process is site and project specific and will depend on the following:
- Flow rate and acidity/metals loadings
- Efficiency of lime usage
- Sludge settling and solid/liquid separation characteristics
- Waste sludge density and disposal site size (volume) constraints
- Sludge stability (residual neutralization capacity)
- Treated water quality
- Capital investment
- Operating and maintenance cost
Table 7-4 contains an indication of the relative performance of some lime neutralization processes based on a few selection criteria.
Selection Criteria |
Conventional HDS |
Cominco Process |
Geco Process |
Tetra (Doyon) Process |
Staged-neutralization |
|
| Efficient lime utilization | X | XX | XX | XX | XXX | |
| Waste sludge density | X | XX | XX | XX | XXX | |
| Sludge viscosity | XXX | XX | XX | XX | X | |
| Sludge stability | XXX | XX | XX | XX | X | |
| Treated water quality | XX | XX | XX | XX | XX | |
| Legend | X | = good | ||||
| XX | = better | |||||
| XXX | = best | |||||
The process principles for the Geco, Tetra, and Staged Neutralization treatment processes are similar and based on intermediate and final pH adjustment. Staged neutralization is better suited to the treatment of ARD with a high iron and sulphate content.
A novel integrated limestone/lime neutralization process was developed at the South African Council for Scientific and Industrial Research (CSIR) (Geldenhuys et al., 2001), as shown in Figure 7-4. The integrated limestone/lime process incorporates the following three process steps:
- Pre-neutralization using relatively inexpensive limestone
- Lime neutralization to a pH target, which is dictated by the treatment targets such as specific metals removal (This step is also designed to precipitate gypsum.)
- Re-carbonation and pH adjustment using the CO2 generated in the first process step
The benefits of the integrated limestone/lime process relate to the efficient use of relatively inexpensive alkali materials and reuse of alkali sludge produced in the process. The use of limestone in ARD neutralization is somewhat limited by the kinetics of limestone dissolution and armouring of the limestone chips/particles by ferric hydroxide compounds.
Many process streams within mineral processing facilities are highly alkaline (i.e., waters from flotation plants). Therefore, excess process waters from the flotation plant could be mixed with ARD for neutralization.
7.5.1.2 Active Treatment – Metals Removal
As discussed in Chapter 2, the metals content of mine drainage varies significantly depending on the following:
- Geology and geochemistry of the mine environment
- Specific ore being mined
- pH and oxidation/reduction potential of the mine water which governs the solubility of metals
- Source of mine water (e.g., drainage from underground workings, runoff from open pit workings, seepage from waste rock dumps, drainage from mill tailings and ore stock piles, spent ore piles from heap leach operations)
- Climatic conditions
The classical approach to metals removal is based on chemical precipitation, formation of solids particles containing the metal precipitates, and separation of the solids from the mine drainage. Metals [M] can form a number of insoluble compounds with anions, such as:
Hydroxides: Mx+ + x OH- → M (OH)x Carbonates: 2Mx+ + x CO32- → M2(CO3)x Sulphides: 2Mx+ + x S2- → M2(S)x
The solubility of metal hydroxides can be used to illustrate the point. Many metals have an amphoteric property, with decreasing solubility up to a threshold pH, above which the metal solubility increases again because of the formation of soluble complexes. The pH corresponding to the theoretical thermodynamic and minimum solubility of some selected metal hydroxides is shown in Table 7-5.
Metal |
pH Corresponding to Minimum Metal Hydroxide Solubility/L |
| Antimony, Sn2+ | ~ 4.2 |
| Ferric iron, Fe3+ | ~ 3.5 |
| Aluminum, Al3+ | ~ 4.5 |
| Lead, Pb2+ | ~ 6.5 |
| Copper, Cu2+ | ~ 7.0 |
| Ferrous iron, Fe2+ | ~ 8.0 |
| Zinc, Zn2+ | ~ 8.5 |
| Nickel, Ni2+ | ~ 9.3 |
| Cadmium, Cd2+ | ~ 10.0 |
| Manganese, Mn 2+ | ~ 10.6 |
Metals removal by precipitation typically involves alkali addition to a target pH for selective removal of the metal of interest. It may also be advisable to pre-oxidize the metal (or metals) before precipitation where a metal can exist in more than one oxidation state. This will assist precipitation because the more oxidized form of some metals has a lower solubility. This is, however, not true for compounds of chromium, selenium, and uranium, which form soluble carbonate complexes in the oxidized state.
A common approach to enhance removal of specific metals is the use of chemical pretreatment or co-precipitation strategies such as the following:
- Aeration can be used to improve removal of iron and manganese.
- In low-iron containing waters, iron may be added to co-precipitate or adsorb certain metals onto ferric hydroxide precipitates. This process achieves lower effluent concentrations than would be achieved solely based on the solubility of the pure metal hydroxide.
- Chemical reduction or oxidation can be used to alter the valence state of a target metal and enhance its removal. Examples of chemical reduction or oxidation include arsenic, selenium, and chromium.
The key considerations in selecting an appropriate reagent for metal precipitation include the following:
- Materials handling considerations, including road/rail transport, bulk storage, make up, and dosing
- Classification of the reagent as a dangerous or hazardous material requiring special precautions in handling and personnel safety
- Availability and reliability of the supply
- Infrastructure and equipment investment cost of reagent handling, storage, make up, and dosing facilities
- Cost of reagent
- Treatment objectives
The specific process arrangement for metals removal is the same as for neutralization – and is often in a lime/HDS configuration with additional chemical feed and control systems. The primary differences are the potential pre-treatment requirements, operation at an elevated pH, and the possible need to reduce the treated effluent pH with acid or carbon dioxide to meet effluent discharge pH requirements.
7.5.1.3 Active Treatment – Chemical Precipitation for Sulphate Removal
The desalination treatment technologies of interest to mine drainage target a small range of salt species, specifically sulphate salts. Mine water may contain a wide range of anionic species but sulphate is typical of many mine drainages and often represents the primary contaminant. Consequently, sulphate removal is an important treatment objective and is also often key in the reduction of TDS.
Some sulphate is removed by gypsum precipitation during neutralization reactions if lime, limestone, or another calcium source is added during water treatment. In addition, a number of precipitation processes have been developed for specific application to high sulphate content mine waters, including the following:
- Barium sulphate process
- Ettringite (Ca6Al2(SO4)3(OH)12•26H2O) precipitation process
The barium sulphate process is based on the addition of a barium salt to re-precipitate sulphate. The insoluble barium sulphate sludge is separated and removed from the main stream process. This barium is recovered from the sulphate sludge and recycled to the main stream process.
The barium sulphate process has not been developed past the development of a pilot scale demonstration process. While the barium precipitation process is very effective, it is challenged by the following:
- The use of an environmentally toxic compound as a treatment reagent
- Generation and handling of a toxic and hazardous gas (H2S)
- Requirement for thermal regeneration and recycle of the barium reagent
Barium carbonate and barium hydroxide have been tested by CANMET in Canada.
Two variations of the ettringite precipitation process (SAVMINTM and Cost Effective Sulfate Removal [CESR]) have been developed and demonstrated. The ettringite process is based on the addition of aluminum hydroxide in a high pH environment resulting in precipitation of ettringite (a hydrated calcium aluminosulphate mineral), as shown below:
6Ca2+ + 3SO42- + 2Al(OH)3 + 38H2O = Ca6Al2(SO4)3(OH)12•26H2O + 6H3O+ The simplified process flow diagram of the SAVMINTM process is shown in Figure 7-5.
The CESR process is similar in concept but CESR uses a proprietary chemical derived from the cement industry to precipitate ettringite. It has the benefit of not requiring the decomposition of ettringite or the recycling of reagents.
While these processes have been demonstrated, neither has been applied to mine drainage projects for full-scale installations to date.
7.5.1.4 Active Treatment – Membrane Treatment
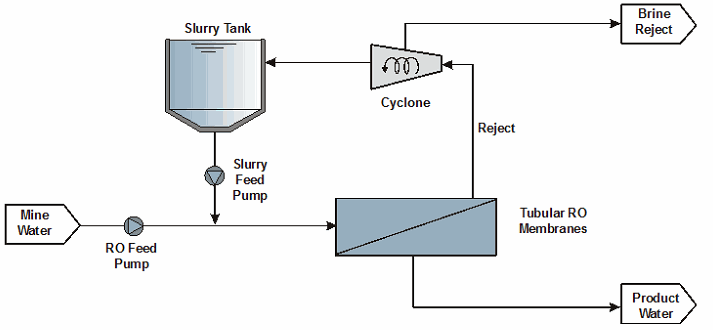
A wide range of membrane treatment technologies exist to treat brackish and saline waters such as mine drainage. The application of these membrane technologies to mine drainage is challenging because of scaling and fouling potential. Mine drainage typically contains several compounds with a scaling and fouling potential such as metals, sulphate, and carbonate. The application of membrane desalination processes to mine drainage also typically results in the production of sludge and brine streams. In recent years, however, a number of high recovery membrane desalination processes have been developed, constructed, and operated at mine sites.
The concept of a high recovery membrane desalination process is shown in Figure 7-6. The primary features of the mainstream membrane desalination process include the following:
- Pretreatment with lime to remove metals and supersaturated gypsum (this is essential to limit the membrane scaling potential of the mine drainage)
- Pretreatment to remove residual suspended solids
- Pretreatment by adjusting the pH to a nonscaling regime and adding anti-scalant reagent
- Membrane treatment typically accomplished using spiral wound reverse osmosis (RO) or nano-filtration (NF) membranes
- Post treatment (a simple process that may only involve stabilization using an alkali such as lime)
A single pass membrane treatment process will typically achieve only a clean water recovery of 60% to 70% for mine waters. The membrane process still leaves a substantial brine stream, which requires treatment. Methods to treat the brine are discussed in Section 7.6. The following two approaches exist to further increase the clean water recovery and decrease the need for brine handling and disposal:
- The brine stream can be desaturated by lime treatment which destroys the anti-scalant action and precipitates any supersaturated salts. A second stage higher pressure RO/NF process is then used to recover more clean water.
- The brine stream can be further concentrated by conventional thermal evaporation/crystallization treatment. These techniques are capital intensive and require substantial energy.
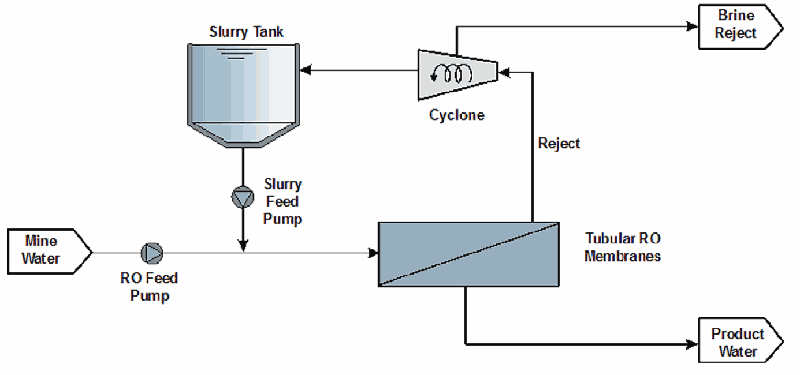
A further variation of the membrane desalination process involves the use of tubular RO type membranes. The Slurry Precipitation and Recycle Reverse Osmosis (SPARRO) process was developed and holds potential as shown in Figure 7-7.
The concept of the SPARRO process is based on the protection of the membrane surfaces by providing a slurry suspension onto which the precipitation products can form. High water recoveries were achieved by a demonstration scale plant (Pulles et al., 1992).
In principle, other membrane processes such as Electrodialysis Reversal (EDR) can also be applied to mine water desalination. No full-scale EDR desalination plants, however, are known to exist in the mine water industry for the large-scale desalination of mine drainage.
7.5.1.5 Active Treatment – Ion Exchange
One of the older ion exchange processes used by mining companies is the copper cementation or precipitation process. In this process, waste galvanized cans were burnt to remove the zinc coating or other metallic iron was placed in a copper containing stream, which was typically leach solution from a waste or low-grade ore pile. Copper in solution would plate on the surface of the iron metal and in doing so would exchange electrons with the underlying iron, oxidizing the iron and reducing the copper to the metallic state. This process created a higher-value product from a waste product (precipitate copper from waste cans) and would reduce somewhat the toxicity of the solution to fish (exchanging Cu+2 for Fe+3 ions), as shown in the following reaction:
3Cu +2 + 2Fe 0 → 3Cu 0 + 2Fe +3
This process has been used to treat copper containing solutions from abandoned mine sites in the United States and it is a process that is amenable to use by artisan miners in developing countries, provided the influent copper concentration is quite elevated (i.e., greater than approximately 20 mg/L).
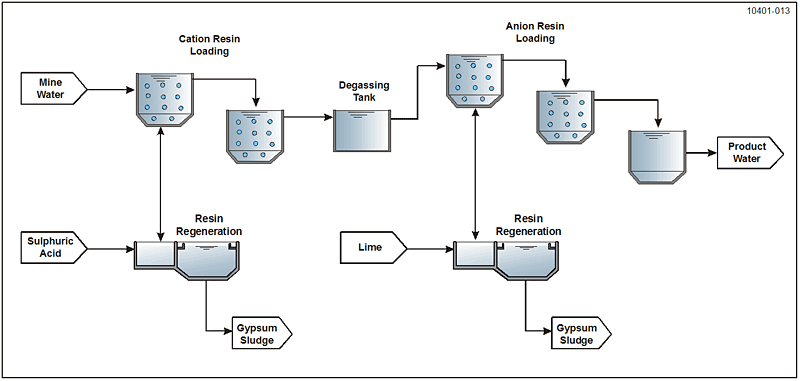
A novel ion exchange process, GYPCIX®, was developed for high sulphate type mine drainage. The process requires pretreatment to remove metals, which may interfere and decrease the efficiency of the downstream ion exchange process resins. The GYPCIX® conceptual flow diagram is shown in Figure 7-8. The cation resin exchanges Ca2+, Mg2+, and other cations (i.e., metal ions) by the following reaction:
2R-H + Ca2+ → R2•Ca + 2H+ The water is acidified in this first process and requires degassing of CO2.
The anion resin exchanges SO42- , Cl-, and other anions by the following reaction:
2R-OH + SO42- → R2SO4 + 2OH-
The product water is near neutral and may require stabilization before distribution or discharge. The resin regeneration requires sulphuric acid and lime, thus producing mainly gypsum as waste sludge.
The GYPCIX process has been demonstrated on a small scale, but no commercial operations exist in the mining industry. A number of natural ion-exchange materials, such as zeolites (a class of aluminosilicate minerals), have been demonstrated to have treatment potential. Few full-scale operating treatment facilities using natural ion-exchange materials exist.
7.5.1.6 Active Treatment – Biological Sulphate Removal
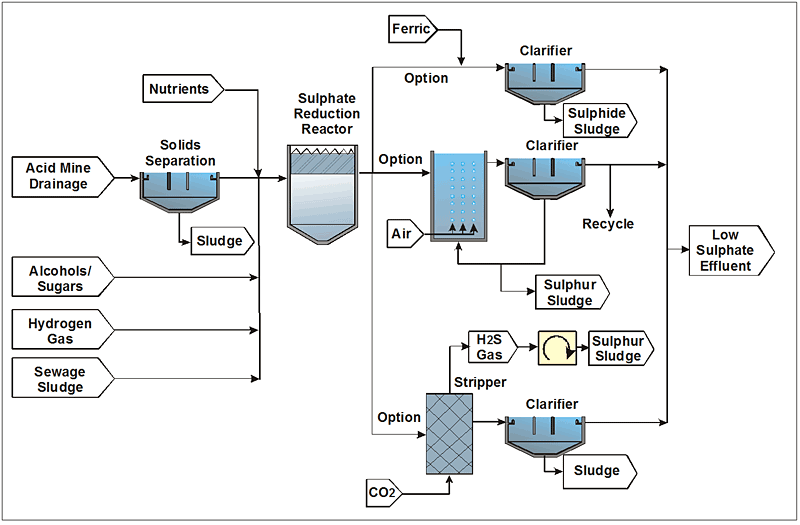
Biological sulphate removal has been used by mining companies at several locations around the world. Many variations of the process have been developed. The generic biological sulphate removal process configuration is shown in Figure 7-9.
The key features of the biological sulphate removal process include the following:
- Pretreatment to remove metals by precipitation as sulphides, hydroxides, or carbonates
- Dosing of an electron donor and carbon source such as alcohol, sugar, H2 gas, and even complex substrates such as sewage sludge
- Addition of nutrients, including sources of nitrogen, phosphate, potassium, and trace minerals
- Sulphate reduction in an anaerobic reactor which converts sulphate to sulphide. The process is mediated by sulphate reducing bacteria (SRB), which uses preferred substrates such as fatty acids, alcohols, and H2 gas. The bacterial population includes a consortium of other organisms such as fermenting bacteria and methanogens, some of which help to hydrolyze and ferment complex carbons to readily available substrates for the SRBs.
The biological sulphate reduction part of the process has been researched and demonstrated by a number of companies. This part of the overall treatment train can be considered as proven technology. The further handling and treatment of the sulphide rich effluent can be done in a number of different ways, as shown in Figure 7-9. A ferric salt can be dosed (or a ferric sludge) to precipitate the sulfide; a ferric sulfide sludge is then generated, which may require special care in disposal and the associated anion may increase salinity of the treated water, as follows:
- The sulfide can be partially oxidized to sulphur in a carefully controlled micro-aerobic environment. The sulphur is separated as a potentially saleable byproduct.
- The sulphide is stripped and converted to sulphur in a side stream process. The substitution of H2S by CO2 results in the increase in carbonate alkalinity and potential precipitation of carbonates such as calcite.
The criteria for selecting an appropriate mine water desalination technology are listed in Table 7-6, with an indication of the relative performance of different technologies.
Selection Criteria |
Mine Drainage Treatment Technology |
|||
Chemical Precipitation |
Membrane Treatment |
Ion Exchange |
Biological Sulphate Removal |
|
| Proven technology on commercial scale | Proven with many demonstration scales, large commercial plants | Proven, with several large commercial plants | Demonstrated on pilot scale, no large commercial plants | Proven, with a limited number of commercial plants |
| Specialized application | General application to high metals, high SO4 mine water | General application, but with appropriate pre-treatment | Demonstrated for CaSO4 type waters, with appropriate pre-treatment | Specialized application to high SO4 mine waters |
| Water recovery | High water recovery > 95% | High water recovery > 90% | High water recovery not confirmed | Very high water recovery > 98% |
| Waste sludge/brine production | Large waste sludge production | Sludge and brine production | Large waste sludge production | Small waste sludge production |
| Potential byproducts recovery | Potential for CaSO4 recovery | Potential, but not demonstrated | Potential for CaSO4 recovery | High potential for Sulphur recovery |
| Chemicals dosing | High chemicals dosing | Limited chemicals dosing | High chemicals dosing | Process depends on carbon source dosing |
| Energy usage efficiency | Moderate energy usage | High energy usage | Moderate energy usage | Moderate energy usage (heating of anaerobic reactors) |
| Reliable and robust performance | Robust process | Process good performance, but sensitive to pre-treatment | IX process performance and resin recovery subject to interference | Biological process sensitive to toxics, fluctuating feed water quality and environmental conditions |
| Capital investment cost (per m3/day capacity) | $ 300 – 1,250 (see note) | $500 – 1,000 | See note | $800 – 1,5-00 |
| Operations and maintenance cost ($ per m3 treated) | $0.2 – 1.5/m3 (see note) | $0.5 – 1.0/m3 | See note | $0.7 – 1.5 |
Note: The cost information on chemical precipitation and ion exchange processes is indicative since no full scale commercial installations exist.
The cost of treating mine waters in cold and remote sites (i.e., arctic regions) could be higher by a factor of 2 or more because of expensive transportation and storage requirements of reagents. These sites are usually accessed by air or ice roads and the treatment systems are installed and operated indoors, requiring construction and maintenance of heated buildings. At closed mining sites, possibilities for seasonal operations should be investigated and applied where possible.
7.5.1.7 Specialized Treatment – Sulphide Precipitation
Sulphide precipitation works under the same basic principle as hydroxide precipitation. The precipitation process converts soluble metal compounds into relatively insoluble sulphide compounds through the addition of precipitating agents, such as the following:
- Sodium sulphide (Na2S)
- Sodium hydrosulphide (NaHS)
- Ferrous sulphide (FeS)
- Calcium sulphide (CaS)
Sulphide precipitation is an effective alternative to hydroxide precipitation. Over a broad pH range, sulphides (S2-, HS–) are extremely reactive with heavy metal ions. Sulphide precipitation can be used to remove lead, copper, chromium (VI), silver, cadmium, zinc, mercury, nickel, thallium, antimony, and vanadium from wastewaters. The precipitation reaction is generally induced under near neutral conditions (pH 7.0 to 9.0). In a way that is similar to hydroxide precipitation, metal-sulphide precipitates most often and must be physically removed from solution (through coagulation, flocculation, and clarification, or filtration), leaving a metal-sulphide sludge.
In addition, sulphide precipitation is sometimes used in water treatment following conventional lime treatment to reduce concentrations of residual metals, particularly cadmium. This is successful because of the ability of sulphide to reduce metal concentrations to much lower values than can be achieved by precipitating metals as hydroxides with lime, although the metals precipitated are not recovered as they report to the lime sludge. Some of the advantages of sulphide treatment include effective metal removal for most metals, low retention time requirement, and reduced sludge volumes. The disadvantages of sulphide treatment are significant and include potential for toxic hydrogen sulphide gas emissions and residual sulphide in treatment effluent. Another disadvantage is that the soluble sulphide process may result in odour problem and more complex systems frequently have higher capital and operating costs than lime treatment.
7.5.2 Passive Treatment Technologies
Passive treatment refers to processes that do not require regular human intervention, operations, or maintenance. It should typically employ natural construction materials, (e.g., soils, clays, and broken rock), natural materials (e.g., plant residues such as straw, wood chips, manure, and compost) and promote the growth of natural vegetation. Passive treatment systems use gravity flow for water movement.
Pulles (2004) formulated a definition for a passive treatment system as follows:
“A water treatment system that utilizes naturally available energy sources such as topographical gradient, microbial metabolic energy, photosynthesis and chemical energy and requires regular, but infrequent maintenance to operate successfully over its design life”
Gusek (2002) also defined passive treatment as:
“.... a process of sequentially removing metals and/or acidity in a natural-looking, man-made bio-system that capitalizes on ecological and geochemical reactions. The process does not require power or chemicals after construction, and lasts for decades with minimal human help”.
A truly passive system should also function for many years without a major retrofit to replenish materials, and should be able to function without using electrical power. Benning and Ott (1997) describe a volunteer passive system at an abandoned lead-zinc mine in Ireland that has apparently been functioning unattended for over 120 years. Similar volunteer systems are likely to be found functioning at some level of efficiency in most historical mining districts.
The generic categories of passive treatment systems are detailed in Table 7-7.
Passive Treatment Technology |
Application Niche in Mine Drainage |
| Aerobic wetlands | Net alkaline drainage |
| Anoxic limestone drains (ALD) | Net acidic, low Al3+, low Fe3+, low dissolved oxygen drainage |
| Anaerobic wetlands | Net acidic water with high metal content |
| Reducing and alkalinity producing systems (RAPS) | Net acidic water with high metal content |
| Open limestone drains (OLD) | Net acidic water with high metal content, low to moderate SO4. |
The proven application of passive treatment technology is to the low-flow range. Most successful passive treatment projects are treating less than 1,000 m3 per day. The largest documented passive treatment system has been treating about 6,500 m3 per day since 1996 with limited maintenance.
Hedin (1994) developed a decision support flow sheet to assist in the selection of an appropriate passive treatment technology. This was further refined by the PIRAMID Consortium, as shown in Figure 7-10 (Piramid Consortium, 2003).
The mechanisms of metal removal and retention in passive treatment systems are varied and include the following:
- Oxidation
- Precipitation as hydroxides and carbonates under aerobic conditions
- Precipitation as sulfides and hydroxy-sulfate (aluminium special case) under anaerobic conditions
- Complexation and adsorption onto organic matter
- Ion exchange with organic matter
- Uptake by plants (phyto-remediation)
The environmental conditions in the different passive treatment systems will dictate the dominant metals removal mechanisms.
The precipitation of iron as hydroxides and carbonates may also assist in the removal of additional pollutants. Several ionic species, such as arsenic and molybdenum, co-precipitate or adsorb onto ferric hydroxides. There is evidence that some of these reactions can be microbially facilitated (LeBlanc et al., 1996).
Sections 7.5.2.1 through 7.5.2.7 provide a brief overview of the principal passive treatment technologies.
7.5.2.1 Aerobic Wetlands
Aerobic wetlands provide the environmental conditions for removal of suspended solids and selected metals using the following features:
- Aerated water surfaces and shallow water depths to allow aeration of the mine drainage
- Configuration and layout to promote favourable hydrodynamic flow conditions (prevent short circuiting)
- Wetlands vegetation to assist in aeration of the substrate (wetlands vegetation has the capability to maintain aerobic conditions around the root/rhyzome area and can also promote favourable flow conditions)
- Cascades to further enhance aeration
- Sufficient residence time to allow the treatment reactions to take place
- Space for the settling and accumulation of the metal precipitates and solids
- Layout and screening against wind mixing and resuspension of settled solids
- Promote algal growth to further increase the pH and facilitate manganese oxidation and precipitation
- Piping and hydraulic controls to manage the water levels in individual wetlands cells
The layout and slope of aerobic wetlands should be designed to minimize disruption of the natural conditions when the wetland sludge is removed and substrate is replaced, while maintaining the above engineering considerations. Any habitat value should reflect the potential uptake of toxic metals to birds, riparian mammals, and amphibians while enhancing the aesthetic quality of the project.
7.5.2.2 Anaerobic Biochemical Reactors
The ARD treatment mechanisms for anaerobic biochemical reactors (BCRs) (also referred to as compost reactors) are based on alkalinity addition using the following two mechanisms:
- Sulphate reduction, which converts SO42- into H2S in an organic rich environment devoid of oxygen, releases alkalinity as a byproduct as follows:
SO42- + 2CH2O → H2S + 2HCO3
- Limestone and dolomitic material react to neutralize acidity as follows:
CaCO3 + H+ → Ca2+ + HCO3-.
Carbonate material also suppresses fermentation bacteria, which are required in the bacterial consortium, but are not desirable in quantity as fermentation byproducts can lower the pH.
The key features of an anaerobic biochemical reactor are as follows:
- A substrate bed containing a varied blend of natural material (e.g., wood chips, crushed limestone, plant residue, grass cuttings, hay, straw, manure, and compost)
- A surface pond (at least 150 millimeters [mm] deep), which floods the substrate bed and limits oxygen ingress into the BCR
- Mine water flow distribution and collection system to promote a plug flow pattern (typically configured vertically) with limited risk of short circuiting or dead zones
- Flow and level control devices to control the water level and to prevent substrate from being exposed to the atmosphere
- Higher plant life may be present to assist with organic material supplementation, as a wildlife habitat and for aesthetic appearance. However, vegetation may need to be suppressed in BCRs with a relatively thin (< 750 mm) substrate layer because the oxygen infusion from the plant activity can impact the establishment of geochemical reducing conditions.
BCRs constructed in the 1990s were typically horizontal plug-flow cells that resulted in a significant amount of mine water flow across the cell surface. The current common practice is to use a vertical flow configuration with untreated water introduced at the top of the cell and treated water collected from the bottom.
The mechanisms of metals removal vary depending on the specific metal, but mechanisms of metals removal are a combination of the following:
- Sulfide precipitation
- Oxidation/hydrolysis (on the BCR surface if iron is present)
- Carbonate precipitation
- Absorption onto organic matter
A key advantage of BCRs is that the organic matter is typically found locally, as is the consortium of bacteria that populate the substrate. Common animal manure (browsing animals like cows, sheep, or goats are preferred) provides the bacterial inoculum for these units. Design life is typically 20 to 30 years before the substrate needs to be exhumed and replaced with fresh materials.
BCRs are typically followed by aerobic cells. Systems are typically comprised of two BCRs to facilitate long-term maintenance (all flow is temporarily directed to one BCR, while the other is being retrofitted) feeding into a single multiple-compartment aerobic wetland.
7.5.2.3 Reducing and Alkalinity Producing System Wetlands
A reducing and alkalinity producing system (RAPS) is a variation to the horizontal flow anaerobic BCR. It is similar in construction to the BCR, but the function of a RAPS is to reduce ferric iron to ferrous in a thin (150 mm) organic layer (as opposed to a much thicker substrate layer in the BCR) and the introduction of alkalinity in a limestone layer (1 meter thick) installed beneath the organic layer. In the case of the RAPS, the treatment concept is based on a vertical flow configuration. Many different RAPS wetland configurations are available with some of the following features:
- Wetland baffling to achieve a vertical flow pattern, thus attaining better contact between the mine drainage and substrate
- Successive layers of different organic substrate and calcitic/dolomitic material
- Sufficient hydraulic head (2 to 3 meters) to drive the flow through the RAPS substrate bed, which has historically been a mushroom compost of low permeability
- Flow distribution and collection to promote a uniform flow across the entire wetland
- Hydraulic and level control devices to maintain a flooded wetland, but with no free standing, exposed substrate surfaces
The mechanisms for release of alkalinity and removal of metals are in essence the same as for the anaerobic wetlands.
These passive treatment systems were originally termed “successive alkalinity producing systems” (SAPS), based on the potential for several units in a series configuration. Most practical applications, however, involve a single system or unit followed by an oxidation pond to precipitate and settle iron from the alkalinity-buffered RAPS effluent. If aluminum is present in the mine water, this alkalinity-adjusting method should be avoided because of potential plugging.
7.5.2.4 Anoxic Limestone Drains
Anoxic limestone drains (ALDs) are designed and operated to introduce alkalinity into an acid mine water. The success of ALDs depends on the following prerequisites:
- Iron must be in the reduced ferrous (Fe II) form because ferric iron (Fe III) will armour the limestone material (if not, use a RAPS, as described in Section 7.5.2.3)
- No free oxygen (< 1 mg/L) must be present; otherwise iron (Fe III) precipitation will take place (see RAPS in Section 7.5.2.3, if water is oxygenated)
- Low mine water aluminum concentration (< 2 mg/L) because any aluminum hydroxide precipitates will clog the limestone bed
The successful application of ALDs is based on having the following features:
- The input mine water in a reduced state, devoid of oxygen (in some cases, this may require pre-treatment in a small anaerobic wetland section to reduce any oxidized iron and strip residual oxygen)
- Limestone with a high calcium carbonate content (> 80%) in size range of 50 to 75 mm
- A hydraulic flow control system to maintain a flooded condition in the ALD
- A cover which may be vegetative or organic rich to prevent any surface ingress of air into the ALD
- A small percentage of inert rock in the limestone mix, so that a hydraulic pathway is continually maintained through the unit for its design life, even if the limestone in a particular zone is completely consumed
- A vent for excess CO2 formed in the ALD
7.5.2.5 Open Limestone Drain
Open limestone drains (OLD) are designed to introduce alkalinity into the dissolution of exposed limestone in the bottom and sides of a limestone drain. The design and operation of the limestone drain require special attention to accommodate the inevitable armouring and coating of the limestone. The following features in open limestone drain are recommended:
- Steep drain slopes of > 20%
- High flow velocities to scour settled solids and clean precipitates from the limestone surfaces
- Ability to periodically flush the OLD and clear accumulated precipitates and solids
7.5.2.6 Passive Sulphate Removal
A special category of passive treatment technology has been specifically developed to achieve high rates of sulphate reduction and ultimately sulphate removal as elemental sulphur. While anaerobic wetlands do incorporate a degree of sulphate reduction, rates are low and these wetlands are without a dedicated oxidative process to remove the sulphides as elemental sulphur. An integrated passive mine water treatment process has been developed in South Africa (Pulles et al., 2004) using integrated and managed passive treatment (IMPI). The IMPI technology has not been applied to many full-scale and permanent treatment sites. Passive sulphate removal uses the same fundamental treatment mechanisms at work in an anaerobic wetland, but with some of the following novel features:
- The Degrading Packed Bed reactor is filled with a specific sequence of selected organic materials, designed to hydrolyze ligno-cellulosic materials. The objective is to sustainably produce volatile fatty acids (VFA) to drive the sulphate reduction process.
- The sulphide oxidizing reactors (primary and secondary) are intended to partially oxidize the H2S to sulphur, with limited impacts on the VFA concentrations.
- The sulphate reduction reactor relies on the upstream generation of adequate and suitable readily biodegradable compounds, such as VFAs to support the sulphate reducing bacteria.
7.5.2.7 Passive Treatment System Performance
The performance and useful life of passive treatment systems are difficult to predict with a high level of confidence. The treatment kinetics and efficiency of such systems are influenced by site-specific environmental conditions, flow conditions and patterns, complex natural organic material, water chemistry, and seasonal variability. It is therefore important to pilot test such technologies before full-scale implementation and to use conservative design criteria and performance estimates.
Design and operation of passive treatment systems must take into account seasonal variations and specifically cold climate winter conditions. All biochemical and microbial reaction rates decrease as the temperature drops. Freezing conditions will impact passive treatment system performance causing the system to fail. Care must be taken in applying the generic design criteria to such cold winter operating conditions. Precautions can be taken in the case of some passive treatment facilities (such as anoxic limestone drains and RAPS) to insulate the treatment unit against the extremes of winter temperatures. However, the mine drainage temperature may still decrease during winter and impact treatment efficiency. Pilot testing over a full year or more should provide data on efficiency changes in response to depressed mine water temperatures, if present.
Limited information is available from full-scale treatment processes operated for a sustained period of time on the removal efficiencies for metals and other mining-associated compounds. Younger et al., (2002) compiled a summary of the postulated passive treatment removal mechanisms, which has been enhanced and modified based on the prevailing wisdom and experience regarding these systems, as shown in Table 7-8.
The removal of these metals and mining-related compounds takes places simultaneously with the mainstream processes of removal of acidity, iron, sulphate, and aluminum, if present. The available information on the removal rates of the nonferrous metals and other mine water parameters is growing, but site-specific verification is highly recommended.
Parameters |
Postulated Removal Mechanisms |
|
Aerobic Wetlands |
Anaerobic Wetlands |
|
| Arsenic | Oxidation to form AsO43-, adsorption to ferric oxides | Reduction to As3+, precipitated as a number of, sulphides |
| Cadmium | - | Precipitation as a sulphide |
| Chromium | - | Reduction of Cr6+ to Cr3+, precipitated as hydroxide |
| Copper | Oxidation in alkaline environment, precipitation as carbonate | Reduction and precipitation as sulphide |
| Cyanide | Photolytic conversion, bacterial oxidation to NH3 and N2 | Reduction and decomposition to NH3 and CO2 |
| Lead | Oxidation in alkaline environment, precipitation as carbonate | Precipitation as a sulphide |
| Nickel | - | Precipitation as a sulphide |
| Zinc | Precipitation as a carbonate | Precipitation as a sulphide |
Passive treatment processes have limited proven capacity for manganese removal, and more research is needed to refine passive manganese-specific treatment systems. Wetland systems are specially designed and operated for manganese removal, and these wetland systems function according to a combination of the following mechanisms:
- Shallow aerobic wetlands with oxic rock media, which support the growth of a bacterial/algal consortium. This environment locally generates high pH and can effectively oxidize and precipitate manganese. The algae employ the MnO2 formed to provide hold-fasts to rocks in the flowing water.
- Pyrolusite Process® based on limestone beds, which are inoculated with Mn-oxidizing bacteria; prior iron removal is recommended.
- Wetlands incorporating dolomite and manganese oxide substrate, which oxidize and precipitate manganese.
- Chitin, which has been used on a pilot scale to remove manganese, (but the mechanisms are not well understood.
Research work is needed to further refine manganese-specific passive treatment systems.
7.5.3 In situ Treatment Technologies
In situ treatment of mine drainage can be undertaken in many different ways and configurations. This Section 7.5.3 is restricted to a brief discussion that includes the following:
- Spreading of alkaline material across mining impacted land and mine waste
- In pit water (pit lake) treatment
- Organic covers of mine land and mining waste
- Permeable reactive barriers (i.e., organic-rich material, zero-valent iron)
In situ treatment of acidic mine water by injection of alkaline lime slurry to disturbed mine land, spoils, and mining waste has met with mixed success for mine drainage. The challenges to practical mine scale applications include the following:
- Flow and transport characteristics of the mine waste material, described as pseudokarstic aquifer due to the presence of interconnected preferential flow paths
- Introducing the lime slurry (or any other alkaline solution) in a manner that will ensure distribution and effective contact with acid producing zones or water bodies
- The scale of such operations and the preparation of infiltration beds or trenches, which do not blind or suffer from ponding
Full-scale trials have been conducted in West Virginia surface coal mines (Donovan et al., 2000) with some success.
Pit lake treatment typically involves the spreading and dispersion of an alkali material across the accumulated water surface. The challenge is to effectively bring the alkali material into contact with the large ARD water body. The challenges are as follows:
- Approach 1 - Spreading of the alkali material in a powder or slurry form across the full aerial extent of the pit lake. This relies on the even spreading of the alkali material and sufficient mixing and contact time between the alkali material and the ARD. Unreacted alkali material will drop to the pit floor, with associated neutralization reaction products such as metal precipitates.
- Approach 2 - Abstracting the pit water and pumping/flowing the water across or through an alkali mix device for blending and dissolution of alkali material. The pit water and alkali blend stream is then returned to the pit for completion of the neutralization reactions, precipitation of metals, and dispersion of the alkali material.
- Approach 3 – Adding alkali material in the early stages of pit flooding as water is entering the pit or workings.
The challenges to in situ pit water treatment include the following:
- Effective contact between alkali material and pit water
- Efficient use of available alkali material
- Long-term dissolution of precipitated metals from the sludge layers
- Poor control of the pH and redox conditions in all parts of the pit lake to achieve the target treatment objectives
7.6 Treatment Residues and Wastes
All mine drainage treatment technologies produce some residues (e.g., sludge, brines, and spent media) or emissions (e.g., gasses). These residues and emissions contain the elements and compounds removed from the mine drainage and the additives and supplements dosed in the treatment process.
No consideration of mine drainage treatment technologies is complete without an understanding of these residues and emissions as it relates to the following:
- Relative production in terms of volumes and masses
- Typical characteristics in terms of chemical composition (e.g., hydroxide, sulphide, and NP) and physical properties (i.e., consistency, volatility, and dewater ability)
- Hazardous classification and rating
- Potential environmental impacts
- Disposal options
The treatment residues can be broadly classified into the following two categories:
- Sludge, which is a slurry or dewatered cake containing precipitates of diverse composition
- Brines, which contains soluble salts in high concentrations
The handling and disposal of sludges must take the following into account:
- Dewatering and compaction ability
- Slurry density – moisture content
- Volume – rate of production
- Metal stability – available alkalinity
- Sludge composition
- Economics
The sludge disposal options include the following:
- Engineered sludge ponds
- Underground mine workings, a specially as a backfill material
- Opencast mine workings
- Codisposal with mine tailings and waste
- Incorporation into rehabilitation covers of mine tailings and waste
- Landfills after amendment with a stabilizing material
Brine disposal is much more challenging and the disposal options include the following:
- Incorporation into a mine waste or tailing stream
- Irrigation and potential cultivation of salt resistant plants
- Solar evaporation ponds, possibly with some wind assisted features
- Discharge and dilution in a sanitary sewer
- Mechanically evaporation and crystallization
- Beneficial use in the cultivation of holophilic algal species of commercial value
7.7 Recovery of Useful Products
A paradigm shift has taken place in the handling and management of treatment residues, such as sludges and brines. The recovery of useful and saleable products is now researched and actively pursued. The recovery of useful products from the treatment process waste streams may include the following:
- Metals recovery
- Supplements for mine land rehabilitation and revegetation, such as CaSO4.2H2O
- Alkali recovery, such as CaCO3
- Building and construction related materials, such as gypsum
- Beneficial use of brine in the cultivation of halophilic organisms, such as algae containing high ß-carotenes and other nutritional supplements
- Recovery of saleable products, such as sulphur and magnesium salts
- Agricultural use (e.g., fertilizer)
- Supplement in cement manufacturing
- Gravel from sludge
- Metal adsorbents in used industrial wastewater treatment
- Pigment (ferrihydrite)
Research and development work is currently being focused in this field.
The incentives driving the recovery of byproducts include the following:
- Reduction of waste sludge and brine products, which require perpetual handling and disposal with associated long-term environmental liabilities
- Generation of a revenue stream to partly or fully offset the ongoing treatment cost
- Contribution to the long-term sustainability of mine water treatment projects
The key aspects of successful byproducts recovery in the treatment of mine drainage are as follows:
- The target byproducts must be selectively removed by minimizing the coprecipitation of compounds that would degrade the quality of byproducts.
- Byproducts recovery, as a project objective, will have an impact on the mainstream treatment process in terms of unit treatment, process selection, and sequence of treatment processes.
- Chemicals (reagents) dosing to the mainstream treatment process must take into account the impact on the potential for and composition of byproducts.
7.8 Treatment in the Context of Mine Closure and Post Closure
The approach to mine drainage treatment during and after closure of mining operations must be placed in context with respect to the following factors:
- Changes in mine drainage flow and quality
- Climate change over the long term
- Long-term operations and maintenance
- Capital replacement cost
- Nonmining water user requirements
- Involvement from nonmining stakeholders
Mine drainage volumes requiring treatment may increase or decrease after mine closure. The opportunities for consumptive on-mine water usage decrease after closure, potentially resulting in increased excess mine drainage volumes. On the other hand, completion of rehabilitation work after closure may decrease the ingress of water into old mining operations, resulting in decreased excess mine drainage.
Management and support for long-term post-closure operation and maintenance of mine drainage treatment facilities may be limited. Passive treatment technologies are therefore considered more beneficial in the post-closure situation than active treatment technologies, where applicable.
Mine planners should consider post-closure water treatment system land requirements in the design of tailings storage facilities and mine waste dumps so that space is available, when needed, and post-closure water treatment does not become a major design constraint that forces the implementation of active treatment technologies. For example, a waste rock dump might be configured in a way that leaves adequate room at the toe for collection and passive treatment of residual seepage. A similar design protocol should be followed for tailings dams and other long-term mine waste facilities that may generate drainage in some cases in perpetuity.
The design life of post-closure treatment facilities should be based on geochemical model predictions of the long-term mine drainage flow and quality.
Replacement of capital infrastructure and equipment items must be taken into account for continued post-closure treatment. Mine drainage flows and associated pollutant loads are typically projected to continue for a considerable period after mine closure. In some cases, this long-term projection for continued treatment may even require a reevaluation of the appropriate treatment approach and technology as research and technology development take place.
Communities and other nonmining economic activities may rely on the long-term availability of mine drainage. Such reliance is not necessarily negative because the transfer of mine drainage treatment facilities to a third party may assist in the sustainability of a post-mining situation. For instance, the Emalahleni Local Municipality in South Africa receives a substantial part of their drinking water supply from a mine water reclamation plant (Gunther et al., 2008).
The early involvement of nonmining stakeholders to identify and implement post-closure beneficial and economic use of mine drainage will assist in developing appropriate treatment infrastructure.
7.9 Evaluation and Selection of Drainage Treatment Technologies
The evaluation of alternative drainage treatment technologies and the selection of an appropriate technology for a specific application require consideration of many of the following factors:
- Technical factors:
- Scale of project
- Location and accessibility of project
- Location within the overall mine water cycle and circuits
- Raw water composition and flow rate
- Fit into the life cycle of the mine
- Proven technology
- Treated water quality requirements
- Reliable performance
- Risks related to implementation
- Operational factors:
- Operations manpower and labour requirements
- Process control and automation
- Utility requirements (e.g., electrical power and water)
- Chemicals and reagents requirements
- Maintenance
- Logistics and communications
- Environmental factors:
- Residual impacts of treated water discharge
- Climatic conditions
- Waste disposal
- Land use impacts
- Regulatory approvals
- Financial factors:
- Capital investment
- Capital replacement costs
- Operations and maintenance (O&M) costs
- Management factors:
- Negotiating with regulators and other stakeholders
- Defining decision process
- Funding for all phases of mining
- Negotiating for unexpected resources requirements
- Maintaining companies’ credibility and good standing
- Social Factors:
- Community acceptance and involvement
A life cycle financial model approach is typically applied to evaluate the treatment project financial implications, including the following:
- Production and management of wastes and emissions
- Potential for byproduct recovery
- Sustainability during active mining and post-closure phases
7.10 References
Beining, B.A., and M.L. Otte, (1997). “Retention of Metals and Longevity of a Wetland Receiving Mine Leachate,” in Proceedings of 1997 National Meeting of the American Society for Surface Mining and Reclamation, Austin, Texas, May 10-16.
Donovan J.J., Fazier, J., Ziemkiewicz, P.F., Daly, M., Black, C., and Werner, E., (2000). Experimental Injection of Alkaline Lime Slurry for In-situ Remediation of an Acidic Surface Mine Aquifer. Paper presented at ICARD (2000).
Geldenhuys AJ, Maree JP., de Beer M., and Hlabela P. (2001). An Integrated Limestone/lime Process for partial Sulphate Removal. Paper presented at the Conference on Environmentally Responsible Mining in South Africa. CSIR, Pretoria. South Africa. September 2002.
Gunther P., Naidu T., and Mey., W. (2008). Emalahleni Mine Water Reclamation Project- Key Learnings. Paper presented at the Water Institute of Southern Africa, Sun City. May 2008.
Gusek, J.J., (2002). “Sulfate-Reducing Bioreactor Design and Operating Issues: Is This the Passive Treatment Technology for Your Mine Drainage?” presented at the Nation Association of Abandoned Mine Land Programs, Park City, Utah, September 15-18. (PT Definition).
Hedin R.S., Narin R.W., and Kleinmann R.L.P. (1994) Passive Treatment of Coal Mine Drainage. Information Circular 9389. Bureau of Mines. United States Department of the Interior. Pittsburgh, PA. Leblanc M., Achard B., Othman D.B., Luck J.M., Bertrand-Sarfati J and Personné, (1996). Accumulation of arsenic from acidic mine waters by ferruginous bacterial accretions (stromatolites).
Piramid Consortium (2003). Engineering Guidelines for the Passive Remediation of Acidic and / or Metalliferous Mine Drainage and Similar Wastewater. European Commission 5th Framework RTD Project EVK1-CT-1999-000021. Published by University of Newcastle Upon Type.
Pulles W., Juby G.J.G., and Busby R.W. (1992). Development of the Slurry Precipitation and Recycle Reverse Osmosis (SPARRO) Technology for Desalination Scaling Mine Water. IAWPRC Specialized Conference on Membrane Technology in Wastewater Management. Cape Town.
Pulles, W., Coetser, L., Heath, R., Muhlbauer, R. (2004). Development of high-rate passive sulphate reduction technology for mine waters. Proceedings of IMWA Conference, 19-23 September (2004), University of Newcastle, UK.
Younger PL., Banwart South Africa and Hedin (2002). Mine Water: Hydrology, Pollution, Remediation. Kluwer Academic Publishers, Dordrecht, Netherlands.
Zinck et al., (2007). Chemical Treatment Options for Effective Sulphate Removal from Acidic Drainage and Process Water. Sudbury paper.
List of Tables
- Table 7-1: Qualitative Comparison of Different Categories of Treatment
- Table 7-2: Alkali Materials and Compounds Applied to ARD Treatment
- Table 7-3: Comparative Table Different HDS Process Configurations
- Table 7-4: Selection Criteria for Lime Neutralization Processes
- Table 7-5: Theoretical Minimum Metal Hydroxide Solubility pH
- Table 7-6: Criteria for Selecting an Appropriate Mine-Water Treatment Desalination Technology
- Table 7-7: Generic Categories of Passive Treatment Systems
- Table 7-8: Postulated Removal Mechanisms of Metals and Mining-related Pollutants in Passive Treatment Systems
List of Figures
- Figure 7-1: Generic Mine Water System Indicating Potential Position for a Drainage Treatment Facility
- Figure 7-2: Generic Range of Drainage Treatment Technologies
- Figure 7-3: Basic HDS Process Configuration
- Figure 7-4: Integrated Limestone / Lime Neutralization Process
- Figure 7-5: Simplified Ettringite Precipitation Process Diagram
- Figure 7-6: Conceptual High Recovery Membrane Desalination Process
- Figure 7-7: Concept SPARRO Process Flow Diagram
- Figure 7-8: Conceptual GYPCIX®ion Exchange Treatment Process
- Figure 7-9: Generic Biological Sulphate Removal Process Configuration
- Figure 7-10: Selection of Passive Treatment Technology Chart