Chapter 5b
- 5.1 Introduction
- 5.2 Objectives of Prediction Program
- 5.3 The Acid Rock Drainage Prediction Approach
- 5.4 Prediction Tools
- 5.4.1 Introduction
- 5.4.2 Geological and Lithological Investigations
- 5.4.3 Hydrogeological/Hydrological Investigations
- 5.4.4 Introduction to Geochemical Characterization
- 5.4.5 Sample Storage and Preparation Prior to Analysis
- 5.4.6 Summary of Testing Requirements
- 5.4.7 Physical Characteristics
- 5.4.8 Total and Near-Total Solid-Phase Elemental Concentration
- 5.4.9 Mineralogical Properties
- 5.4.10 Net Acid or ARD Potential
- 5.4.11 Short-Term Leach Tests
- 5.4.12 Laboratory Kinetic Tests
- 5.4.13 Field Methods
- 5.4.14 Data Management
- 5.4.15 Quality Assurance/Quality Control
- 5.4.16 Screening and Evaluation Criteria
- 5.4.17 Reporting
- 5.5 Modeling of Acid Rock Drainage, Neutral Mine Drainage, and Saline Drainage for Characterization and Remediation
- 5.6 Conclusions
- 5.7 References
- List of Tables
- List of Figures
- First Page: Sections 5.1, 5.2, and 5.3
- This is the Second Page: Section 5.4 Prediction Tools
- Third Page: Sections 5.5, 5.6, and 5.7, Lists of Tables and Figures
5.4 Prediction Tools
5.4.1 Introduction
This section describes the main methods of estimating the environmental water-quality consequences of mineral extraction and processing and how these tools could be used to aid in remediation planning and remedial action. These tools build on the approaches described in Chapter 4.
The primary prediction tools discussed in this chapter include the following:
- Geological and lithological investigations
- Hydrogeological investigations
- Geochemical testing methods
- Laboratory static and short-term methods
- Laboratory kinetic methods
- Field methods
- Modelling
5.4.2 Geological and Lithological Investigations
Mineral deposits are categorized according to their temperature of origin, their mineralogy, their lithology, and their structure. These categorizations are the basis for the development of geo-environmental models described in Chapter 2. A thorough understanding of the mineral deposit is critical to the characterization of mine wastes and geologic materials and the prediction of mine drainage quality. This information is typically available from the project geologist. Therefore, the characterization and prediction programs often begin with assembly of geological reports and interviews with the project geologists.
The elements likely to be of concern in water-quality assessments have a source in the rock and minerals that are exposed to weathering because of mining activities. Qualitative predictions on what those elements are can be gained from the rock type, its type and degree of alteration (e.g., hydrothermal, weathering, metasomatic), and the structural controls, including those that affect permeability and surface and groundwater flow. Examples of important geological characteristics that can affect the drainage quality, and hence the characterization program, include the following:
- The presence of a pyrite halo around the mineralized zone
- The role of alteration (e.g., potassic vs. propylitic vs. quartz-sericite-pyrite alteration in porphyry copper deposits) in the presence and distribution of sulphide and carbonate minerals
- Vein vs. disseminated deposit
- The presence and role of faults in displacing mineralized and nonmineralized zones and as conduits for water
- Depth of weathering (e.g., supergene vs. hypogene alteration)
- Sedimentary/stratigraphic sequence of coal deposits
These factors will ultimately determine the chemical composition of the mine drainage source material, which is an important step toward predicting the chemical composition of the mine drainage. An example of geological information that is relevant to ARD prediction and can be gathered by mine geologists during their exploration programs is presented as Table 5-2.
Quantitative Data:
- Visual sulphide content (primarily pyrite) with an estimate of accuracy
- Visual carbonate content with an estimate of accuracy
Semi-Quantitative Data:
- Mineralogy, grain size, mode of occurrence of sulphides
- Mineralogy, grain size, mode of occurrence of carbonates
- "Fizz" reaction of carbonates (strong, weak, none - powdered and unpowdered)
- Extent of oxidation, if any, of rocks
- Presence of gypsum, barite, graphite or siderite
- RQD or other tests of rock competence
- Limit of oxidation and supergene zones
- Presence of water (depth to water table)
- Rock hardness/competence
Qualitative Data:
- Presence of secondary sulphate minerals and identification where possible
- Weathering or slaking potential (unusual observations such as rapid oxidation or weathering) in core as recovered or after storage
- Potential for breakage along fracture planes and for preferential exposure of sulphides and/or carbonates
- Presence of coating on sulphides and carbonates
- Potential problems in collecting samples for analysis and testing (e.g., core loss, concentration of holes near ore versus waste, lack of core at depth, difficulty visually segregating different geological units, differences in specific gravity, biasing by sulphide/carbonate stringers, etc.)
- Observations at outcrops of deposit (sulphide/carbonate content, extent of weathering, staining, coatings, etc.)
- Presence of staining or precipitation in streams or seeps draining the deposit
Quantitative data should be compiled for each drill interval and entered into a geologists log. Semi-quantitative information should be collected periodically through the core when significant changes are noted and could be entered into the "comments" section of log records. Qualitative information relates to unusual conditions that may be encountered while logging or storage of the samples and could be described in a covering memo from the exploration geologist. Geology staff should also advise environmental staff and ARD/ML consultants of any samples submitted for whole rock, metal scans, mineralogical or petrographic analysis as this information is often also relevant to ARD/ML prediction.
5.4.3 Hydrogeological/Hydrological Investigations
Contaminants in surface water and groundwater result from hydrologic and geochemical processes. The conceptual site model (as discussed in Chapter 4) of the hydrologic system includes recharge (precipitation, snowmelt, infiltration, minus evapotranspiration), flow paths, and discharge (springs, abstraction boreholes, seeps, portal flow, and base flow to a river or stream). These water fluxes should be estimated (flux-reservoir diagram) and pump tests are usually needed to determine the geohydrological characteristics of aquifer material. Often a potentiometric surface for underground workings, waste piles, and open pit or other excavations needs to be estimated to determine the current or future potential conditions for water flow and changes in direction of that flow. Determining the groundwater table in fractured rock terrain with or without mine voids (i.e., an open pit or underground mine) can be challenging but very useful information, even in a rudimentary form.
5.4.4 Introduction to Geochemical Characterization
Geochemical characterization requires careful sampling (Section 4.3.2.1), sample preparation (Section 5.4.5), analysis and testing (Sections 5.4.6 to 5.4.13), data management (Section 5.4.14), quality assurance and control (Section 5.4.15), and data interpretation and use (Section 5.4.16 and 5.5). Sections 5.4.5 to 5.4.16 describe characterization methods and how the test results can be used for prediction of ARD and drainage chemistry. Possible outcomes of geochemical testing include identifying materials suitable for construction uses, as a medium for plant growth, and options for the mining sequence, material handling, waste disposal, and mitigation.
This section represents a high-level overview of available test methods rather than a detailed account of individual procedures, and focuses on the interpretive and predictive value resulting from geochemical tests. Table 5-1 provides a summary description of various test methods used globally and brief discussions of advantages and limitations of the test methods.
Figure 5-5 (Maest and Kuipers, 2005) schematically presents the components of a typical geochemical characterization program aimed at developing water quality predictions and the general sequence in which these components should be conducted. This flowchart in Figure 5-5 provides more detail on the Phase 1 and Phase 2 testing programs illustrated in Phase 1 consists of a screening-level program, while Phase 2 is more detailed. In some cases, a Phase 1 program may be sufficient for mine water and waste management, whereas in more complex settings, a Phase 2 program is generally required. When a Phase 2 program is required, the results from the Phase 1 program are used to identify samples for kinetic testing or additional static testing, such as those identified in Figure 5-1 and Figure 5-5.
Therefore, not all components of the geochemical testing program may be necessary depending on site-specific characteristics and prediction needs. Individual test methods are described in more detail in the Sections 5.4.7 through 5.4.13, and are summarized in Table 5-1. Not all test methods presented in the table are appropriate for evaluation of mine wastes, even though they occasionally are requested by regulatory authorities. Such methods include the Toxicity Characteristic Leaching Procedure (TCLP) and Waste Extraction Test (WET), as explained in more detail in Table 5-1.
(modified from Maest and Kuipers, 2005)
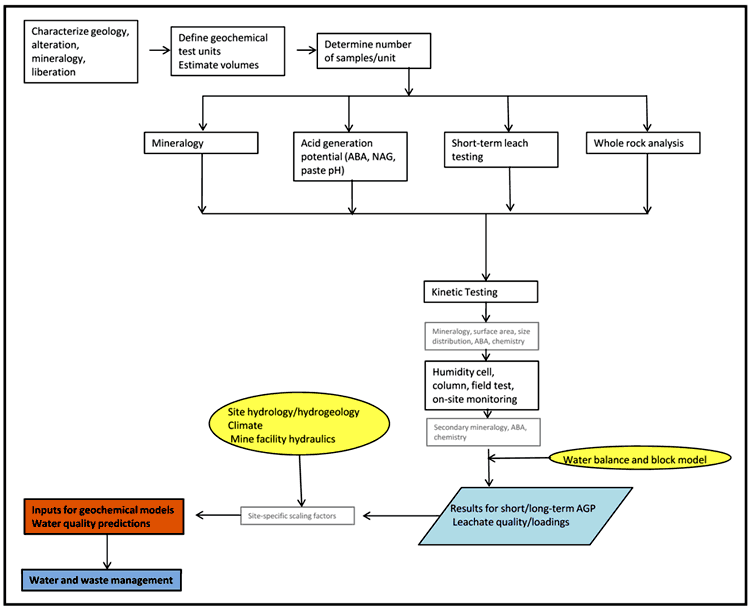
The geochemical characterization program starts with bench-scale testing, which generally involves whole rock analysis to determine chemical composition. In addition, mineralogical examination, evaluation of acid generation potential, and evaluation of metal leachability are used to determine the ARD/ML potential. Detection limits in tests must be low enough to measure contaminants at potential concern levels. Depending on the complexity of the geology and variation in ARD potential, the results from the acid generation testing might be combined to develop a 3-dimensional representation of the quantity and geochemical characteristics of ore and waste rock. The information from the whole rock analysis is used to identify categories of rock in support of development of a waste management plan, which aims to handle mining wastes in such a manner as to prevent or minimize environmental impacts (see Chapters 6 and 9).
The next important step in the geochemical characterization program is kinetic testing, which can take the form of laboratory testing, field testing or both laboratory and field testing, supplemented by on-site water quality monitoring. All materials involved in the kinetic testing should undergo a comprehensive characterization before the test begins, including surface area, particle size distribution, mineralogy, chemical composition, acid neutralization potential, and acid generation potential. At the completion of kinetic testing, the interpretive value of the kinetic testing program is greatly enhanced by repeating the determination of mineralogy, chemical composition, and acid generation potential.
In combination with water, and sometimes oxygen flux calculations, the results from the geochemical characterization programs are used to generate predictions regarding short-term and long-term acid generation potential, leachate quality, and loadings from individual waste type units. These predictions can be extrapolated to full-size mine facilities by incorporating a site-specific water balance based on information on hydrology, hydrogeology and climate, and a block model. Use of scaling factors may be required to account for differences in mass, surface area, rock to water ratio and temperature between testing arrangements, and mine facilities. The resulting water quality estimates can be used as inputs to geochemical models to account for geochemical processes that may affect dissolved concentrations such as mineral precipitation and dilution, sorption, and interaction with atmospheric gases. Ultimately, the findings of the geochemical characterization program contribute to development of mine waste and water management plans.
Any water quality prediction program needs to be customized for a particular situation and problem. Depending on the mine phase, commodity, climate, or mine facility, all or a subset of geochemical characterization tests may be required for the prediction effort and, although not indicated in Figure 5-5, multiple iterations may be required. Water transport might outweigh drainage chemistry as the primary factor determining environmental performance in very arid or arctic conditions with limited or infrequent generation of mine discharges. In that case, the primary focus of the program might be on determining site hydrology and hydrogeology, or the hydraulics of the mine facility rather than the range of geochemical characteristics.
Contaminant loading in drainage discharge is usually the primary prediction concern. Other concerns in the prediction of drainage chemistry may be site reclamation, contaminant loss by wind-born sediment and contaminant uptake by flora and fauna. The ARD/ML potential of material that will comprise a growth medium needs to be determined because of its importance for reclamation and contaminant uptake by flora and fauna.
In general, the earlier in the life of a mine, the greater the reliance on use of laboratory tests for water quality prediction. As the mine matures, use of direct field measurements of material geochemistry and from water quality monitoring becomes feasible and is advocated. Accordingly, the comprehensive characterization program presented in Figure 5-5 is most appropriate for proposed operations, while characterization at inactive or orphaned mines would instead focus on observations regarding existing site water and soil quality.
5.4.5 Sample Storage and Preparation Prior to Analysis
Storage and preparation of samples prior to analysis plays an important role in achieving accurate data and needs to be carefully planned. This section provides an overview of these activities. A more detailed description is provided in Price (2009) (http://www.mend-nedem.org/reports/files/1.20.1.pdf). The objectives of sample storage and preparation are to preserve properties critical to the prediction of drainage chemistry and provide suitable test material for planned analyses and tests. Before samples are collected, a protocol should be developed that outlines the storage and pretreatment requirements for each type of sample and analysis and test. Every sample should be provided with a name, number and a brief description that can be used to identify the sample in the field, laboratory, and during data evaluation. The sample description should include the following:
- Sampling date
- Sampler’s name
- Sampling location (GPS coordinates)
- Area, volume or length over which each individual sample is collected or sub-samples are composited
- Sample size
- Geologic material
- Waste material and project component
- Type of material sampled (e.g., drill core)
- Subsequent treatment, storage, and preparation (e.g., drying and sieving)
- Visual characteristics such as Munsell colour, degree of weathering, mineralogical composition, texture, and particle size distribution
Sample storage conditions should prevent further weathering, especially sulphide oxidation. The most common method to prevent further sulphide oxidation after sampling is drying the sample. Drying temperatures below 40C will ensure most minerals are not altered. Prior to and after drying, samples should be kept cool, and humid storage conditions should be avoided. Where necessary to preserve anaerobic conditions, samples should be stored under nitrogen gas. Freezing can be used to prevent various weathering reactions.
The most common forms of sample preparation are sieving, crushing, and/or grinding. The decision about whether to separate different particle size fractions and crush and/or grind samples depends on the type of sample, logistical constraints, and analysis objectives. Different forms of pretreatment may be required for bedrock (e.g., drill core or chips) versus non-lithified materials (e.g., tailings and waste rock) or measurement of total solid-phase composition versus the soluble chemical species on solid-phase surfaces. Where more than one pretreatment protocol is required, sub-samples can be created using an appropriate method such as a splitter box or coning and quartering.
Sieving may be required to separate the reactive size fraction of non-lithified (particulate) samples. Particulate samples containing stones may be dry sieved into coarser and finer fractions to determine the composition of the more reactive, finer size fraction or to remove particles that are too large for the analysis containers. The weight of each size fraction should be measured, so analytical results can be extrapolated to mine facilities as a whole.
The “reactive” particle size fraction depends on site-specific factors such as the grain size of reactive minerals, previous weathering, and the porosity of the coarse fragments. Based on observations of mineral reactivity made on waste rock with a wide range in grain size, Price and Kwong (1997) recommended that, in the absence of a site-specific evaluation, the minus 2 mm particle size be used as the cut-off for the smallest, more reactive, particle size fraction. The influence of coarse fragments on drainage chemistry increases if coarse fragments break down rapidly, are porous, or the minus 2 mm fraction is unreactive. The assumption that most contaminant releases come from the minus 2 mm fraction may not be correct for historic mine wastes and naturally weathered materials in which weathering has removed reactive minerals from the finer particles.
Many laboratories automatically crush and grind samples to < 74 µm (200 mesh) or < 120 µm (120 mesh) as part of the standard pretreatment without considering whether this will prevent accurate material characterization and the prediction of the drainage chemistry. Whether to crush and grind samples and to what particle size will depend on the sampled material and the proposed analyses and tests. Depending on the laboratory, crushing and grinding to < 74 µm (200 mesh) or < 120 µm (120 mesh) is usually recommended for sub-samples analysis of total elements, sulphur species, neutralization potential and other bulk, whole or total assays. Bedrock samples are often crushed to < 9.5 mm (3/8 inch) or 6.4 mm (1/4 inch) for static solubility water extractions, laboratory humidity cell and column kinetic tests.
Since crushing and grinding creates new particles and surfaces, it should not be conducted on samples of particulate materials prior to sieving, or on sieved particulate material prior to the measurement of surface properties such as rinse pH or soluble constituents produced by surface weathering.
5.4.6 Summary of Testing Requirements
In summary, the evaluation of mine waste ARD/ML potential and prediction of resulting water quality requires an understanding of the following characteristics of the mining wastes and geologic materials:
- Physical characteristics
- Chemical characteristics
- Mineralogical characteristics
- Acid neutralization potential
- Acid generation potential
- Leaching potential
For ease of presentation in this GARD Guide, tests aimed at determining acid generation potential and leaching potential are categorized as follows:
- Laboratory static and short-term methods
- Laboratory kinetic methods
- Field methods
Sections 5.4.7 through 5.4.13 present a brief overview of the components of a comprehensive geochemical characterization program and their significance for mine water quality prediction. Useful references related to static and kinetic testing methods and their interpretation include AMIRA (2002), BCAMDTF (1989), Jambor (2003), Lapakko (2003), Maest and Kuipers (2005), Mills (1999), Morin and Hutt (1997), Price (1997), USEPA (2003), and White et al. (1999).
5.4.7 Physical Characteristics
The physical characteristic of most significance for water quality prediction is the particle size. Particle size distributions impact both mineral reaction rates and reaction duration by affecting the reactive surface area, the distances between potentially reactive particles, and the porosity and permeability of a solid. Porosity and permeability of a solid are particularly important with regard to movement and transport of air, water, and reaction products from weathering reactions.
The particle size distribution should be measured before any kinetic testing, both for laboratory and field-scale tests. To enable scale-up of test results, estimates of particle size distribution in mine facilities, such as waste rock repositories and heap leaches, are also required. These can be determined from direct measurement or estimated from the blasting plan. The “reactive” surface area of a material (i.e., that portion of the total surface that is actively available for chemical reaction) may be significantly smaller than the surface area as measured by standard techniques.
Permeability, specific gravity, and porosity should be determined in the laboratory for tailing material. The soil water characteristic curve (SWCC) and air entry value for oxygen diffusion might also be determined in the laboratory (see Chapter 6).
5.4.8 Total and Near-Total Solid-Phase Elemental Concentration
This section provides an overview of the measurement of total and near-total solid-phase elemental concentrations, which has numerous uses and is a valuable part of drainage chemistry prediction. A more detailed description is provided in Price (2009) (http://www.mend-nedem.org/reports/files/1.20.1.pdf).
Uses for total solid-phase elemental include:
- Identification of materials with elevated concentrations of constituents of potential concern
- Aid in the selection of samples for kinetic testing and interpretation of the results
- Prediction of the maximum concentration of acid insoluble sulphate and trace metal sulphide minerals in ABA
- Identification of anomalous geochemical conditions
- Verification of lithology and mineralogy
Whole-rock or near-total solid phase elemental analysis should be conducted on all impacted geologic materials. Total element data initially originate from geochemical exploration. More comprehensive data are usually collected as part of pre-mine planning, with data from operational characterization used for verification and filling data gaps. Solid-phase analysis consists of two steps: (1) sample digestion and (2) elemental analysis. More detail on these two components of solid-phase analysis is provided in the next two sections.
5.4.8.1 Sample Digestion
The purpose of digestion is to release elements from minerals into a phase in which they can be analyzed. Many digestion and analysis methods are acceptable. A hot chemical flux produces a fused glass disk. Combinations of acids produce a liquid solution. Digestion methods vary in their ability to digest different minerals, susceptibility to interference by sample properties such as sulphide content, and detection limits of the subsequent analyses.
Lithium borate fusion completely digests most samples and is recommended if the objective is to measure the total concentration of major mineral forming elements (i.e., whole rock). The resulting fused disk can be analyzed directly by X-ray fluorescence (XRF) or re-dissolved and analyzed by inductively coupled plasma (ICP). Prior analysis is needed to detect samples where elevated sulphide may interfere with the fusion or require additional dilution before the trace element analysis is conducted. Sodium peroxide fusion rather than lithium borate fusion is used when the sulphide mineral concentration is greater than 5%. Four acid (hydrofluoric, perchloric, nitric, and hydrochloric acid) digestion is the most powerful wet acid dissolution procedure in common use and is considered a near total digestion. Although the lower digestion temperature makes it less able to digest silicates than fusion methods, the four acid method is capable of dissolving most metal salts, carbonates, sulphides, silicates, and almost all sulphates and oxides. Three acid digestion differs from four acid digestion by not using hydrofluoric acid, which makes the digestion of silicates less complete but removes operational challenges associated with the use of hydrofluoric acid.
Aqua regia (3:1 mixture of hydrochloric and nitric acids) is an effective solvent for most base metal sulphates, sulphides, oxides and carbonates, but provides only a partial digestion for most rock forming elements and elements of a refractory nature. It is typically less expensive and does not provide as complete a digestion as the four acid method. However, aqua regia provides a good measure of trace elements in most reactive minerals.
5.4.8.2 Elemental Analysis
Inductively coupled plasma (ICP) measurements are made on liquid samples produced by acid digestion. ICP is capable of measuring 40 to 70 elements simultaneously with relatively high level of detection. The standard ICP procedure for near-total solid phase analysis is ICP atomic emission spectroscopy (ICP-AES). ICP mass spectroscopy (ICP-MS) may measure different ionic species and has lower detection limits than ICP-AES. Low detection limits are rarely needed for solid phase and are primarily used for water samples.
Atomic absorption spectroscopy (AAS) measurements are also made on liquid samples produced by acid digestion. AAS is only capable of one element at a time but the equipment is less expensive. AAS with a graphite furnace has similar accuracy to ICP-AES.
The most common use of XRF is to measure major elements (e.g., Al, Ba, Ca, Cr, Fe, K, Mg, Mn, Na, P, Si, and Ti) in a lithium borate fused disk. Trace elements (e.g., As, Ba, Cu, Ni, Sn, Sr, U, W, Zn, and Zr) are measured in an undigested pressed pellet. Major cations are commonly reported as oxide equivalents (e.g., Al2O3 and MgO). Portable and hand held XRF equipment is increasingly being used for field characterization of undigested samples. Primarily developed for exploration, field XRF measurement of selected elements may be used to identify wastes requiring segregation during waste handling (Guerin et al., 2006). The level of detection in field XRF will depend on sample preparation and the type of XRF equipment. Other total element analysis methods include Leco furnace for carbon and sulphur, gravimetric and volumetric methods, and specific ion electrodes. In gravimetric and volumetric methods, elemental concentration is calculated from the amount of reacting species required to completely react with the element of interest.
Detection limits for total and near-total solid-phase elemental analysis vary between laboratories due to differences in sample preparation, instruments, techniques and range in standards. Detection limits vary between samples due to differences in composition and interferences.
5.4.8.3 General Comments
The most commonly used methods are wet acid digestion by four acid and aqua regia, followed by ICP-AES. Where the objective is to determine the concentration of major mineral forming elements, digestion by lithium borate fusion with analysis by XRF or ICP-AES is recommended.
Whole rock and near-total solid phase elemental analysis does not distinguish the form (e.g., mineral) in which the elements exist. Therefore, this analysis is not on its own a measure of potential elemental concentrations in drainage or the threat to the environment; information on the mineralogy, geochemical conditions and drainage chemistry is needed to predict the environmental significance of solid-phase elemental analysis results.
Different methods of digestion and analysis may produce different total solid-phase results from the same sample. Beware when comparing data from different methods. Methods of digestion and analysis and detection limits must be reported when communicating results, to indicate the potential limitations of the data.
5.4.8.4 Calculation of Mineral Concentrations from Elemental Data
Total element data or selective extraction of different solid-phase fractions (Chapter 11) can be used to calculate maximum potential concentrations of individual minerals by assuming elements occur in only that one mineral phase. This technique is used in ABA to determine maximum concentrations of sulphur that could occur as acid insoluble sulphate (e.g., barite and anglesite) or associated with different sulphide minerals (e.g., Zn in sphalerite and Ni in pentlandite) with equations such as the following:
- Barite [BaSO4]: % Ba x (32.07/137.3) = % Barite-S
- Anglesite [PbSO4]: % Pb x (32.07/207.2) = % Anglesite-S
- Sphalerite [ZnS]: % Zn x (32.07/65.37) = % Zn-S
- Pentlandite [NiS]: % Ni x (32.07/58.7) = % Ni-S
The accuracy of these calculations depends on the accuracy of the assumptions that the element only occurs in one specific mineral phase and the expected elemental composition of the mineral phase. Assuming the elemental composition of the mineral phase is correct, the calculation provides the maximum potential concentration for that mineral phase. Assumptions about the mineral source for specific elements and the elemental composition of mineral phases should be verified using mineralogical tests if these mineral species are potentially important.
Calculation of mineral concentrations from elemental data can range from the relatively simple calculation of individual minerals to complex calculation of an entire mineral assemblage using normative computer programs. Normative calculations produce idealized mineral assemblages from whole rock elemental data, based upon assumptions about the potential mineral phases, order of mineral formation and simplified mineral formulas.
The normative calculation in most common use is the Cross, Iddings, Pirsson and Washington (CIPW) Norm. There are a number of assumptions in the CIPW Norm that deviate from conditions commonly observed in mined geologic materials. These assumptions include no hydrous minerals (e.g., muscovite, hornblende and biotite), ferromagnesian minerals are free of Al2O3, no weathering or hydrothermal alteration, and limited carbon concentrations. Generic normative calculations are, therefore, unlikely to provide an accurate prediction of the mineral assemblage in mined geologic materials and should never be used without detailed mineralogical testing for each geologic unit to verify their accuracy.
5.4.8.5 Comparison with Concentrations in Non-Mineralized Rock
Comparison with concentrations (mg/kg) in non-mineralized rock (e.g., crustal abundance, composition ranges for specific lithologies and soils) can be used to identify the degree to which trace elements concentrations are elevated. The soluble or leachable proportion of constituents of interest can be determined by combining the results from the chemical analysis with those from leach tests.
One measure of enrichment of elements in whole rock samples is the Geochemical Abundance Index (GAI). The GAI compares the actual concentration of an element in a sample with the median abundance for that element in the most relevant media (such as crustal abundance, soils, or a particular rock type). The main purpose of the GAI is to provide an indication of any elemental enrichment that may be of environmental importance. More detail on the use of the GAI is presented here: Elemental composition of mineralized rocks.
Other uses of chemical analyses include evaluation of sample representativeness and determination of all or part of the bulk mineralogy. Chemical analyses may also provide a surrogate for acid base accounting parameters (e.g., Ca for NP; total sulphur for AP). Table 5-3 is an example table of results from chemical analysis of various rock types, including a comparison against crustal values.
5.4.9 Mineralogical Properties
Mineralogical analyses measure properties of individual crystalline and amorphous mineral phases and their contribution to geologic materials as a whole. Mineralogical information is an essential component of drainage chemistry prediction because mineralogical properties determine the physical and geochemical stability and reaction rates of geologic materials and mine wastes. This section provides an overview of the determination of mineralogical properties. A more detailed description is provided in Price (2009 - http://www.mend-nedem.org/reports/files/1.20.1.pdf).
Information about mineral phases potentially required from a mineralogical assessment includes:
- Type and quantity
- Elemental composition (major components and impurities)
- Grain size, crystal shapes and inclusions
- Spatial distribution and associations
- Surface exposure and deformities
- Mode of formation
- Degree of previous weathering and location, size, abundance and elemental composition of weathering products
The type of mineral phase indicates the major chemical constituents and relative reaction rates under different weathering conditions. Surface exposure, grain size and deformities also affect the rate of weathering. One of the most important uses of mineralogical data is to support selection and design of other tests and interpretation of their results. Mineralogical analysis is usually required for a ‘representative’ sub-set of the static test samples and each kinetic test sample.
Comprehensive, accurate and precise mineralogical information may be difficult to obtain. Mineralogical techniques differ in speed and accuracy, and the mineral phases, properties and grain sizes they can measure. It is important to use mineralogical techniques capable of providing the required information.
Challenges associated with mineralogical analysis include:
- Many mineralogical analyses only provide qualitative or semi-quantitative data, or measure a very small sample volume
- Important minerals, such as calcite or pyrite, may occur in trace amounts, making it difficult to detect them, and to measure their concentration and chemical composition
- A significant proportion of potentially important minor and trace elements may be present as impurities rather than major structural elements
- Many minerals are solid solutions (i.e. display a compositional continuum between two end-members) and differences in composition significantly impacts their weatherability and contribution to drainage chemistry. (For example, the mineral “plagioclase” ranges in composition from relatively rapid weathering calcic plagioclase [anorthite] to much slower weathering sodic plagioclase [albite]).
The most commonly used mineralogical procedures are:
- Visual description
- Petrographic analysis (thin section or polished section)
- X-ray diffraction
- Electron microprobe (EM)
- Scanning electron microscopy/energy dispersive spectroscopy (SEM/EDS)
- Laser ablation and other specialized methods
At a minimum, one usually needs to conduct the first two procedures and either number 3 or 4. Other methods, such as microprobe, QEMSCAN® and laser ablation, will be used to answer specific prediction questions.
In addition to the choice of procedure, reliable and useful mineralogical information depends on analyzing samples representative of the geochemical variability and material of concern and adequate care in sample storage and preparation prior to analysis. Representative samples are identified from previous analytical work and a good understanding of the deposit geology. More detail on individual techniques is provided in the following sections.
5.4.9.1 Visual Description
Visual descriptions provide information about large-scale mineralogical variability. Visual descriptions will aid in the extrapolation of small-scale microscopic or submicroscopic mineralogical measurements to project components and geological units as a whole.
Visual descriptions usually come from logging drill core. At existing mines, visual descriptions may be made along transects set up along different mine components. Visual descriptions are commonly made with the aid of a hand lens, hydrochloric acid (HCl), and scratchers, and provide valuable information about:
- Rock type
- Geological variability
- Mineral abundance and association
- Mineral alteration and weathering
- Presence of carbonates (HCl fizz)
- Organic C and S
Users of visual descriptions should be aware of the limitations in visual mineral identification and the tendency to include educated guesses, which are not identified as such (e.g., all carbonate is calcite). While it can provide a good start, visual mineral identification will not be sufficiently accurate for most aspects of drainage chemistry prediction. In addition, an assessment of mineral abundance is generally limited to a qualitative estimate (e.g., trace, minor, major). Comparisons between visual estimates and measured values have demonstrated that quantitative assessment of mineral abundance by visual means tends to be approximate at best, even when conducted by experienced practitioners.
5.4.9.2 Petrographic Microscope Analysis
Petrographic microscopes are used to make measurements based on the optical properties of mineral phases in a translucent or opaque, thinly ground (~ 30 µm) slice of material mounted on a glass slide. Most minerals are identified with transmitted plane-polarized light. Sulphide and a few other minerals are identified with reflected light. Thin sections may be created from rock, chips, pulverized or sieved samples. Thin sections should be polished to allow mineral identification with reflected light and subsequent SEM/EDS analysis.
Sample storage should limit oxidation prior to slide preparation and analysis. Friable and fragile materials, such as secondary minerals, clays and weathering products, require impregnation with resins prior to sectioning. Wet or damp samples must be dried prior to impregnation. Drying should not occur at high temperatures because clay-rich materials and certain sulphates react adversely to heat and water. Thin sections may be impregnated with calcium or potassium specific stains to distinguish between calcic and potassium minerals (e.g., feldspars).
Advantages of petrographic versus sub-microscopic mineralogical techniques include the preservation of individual grains and their spatial distribution and the larger field of vision. Petrography is useful for identifying and measuring (Thompson et al., 2005):
- Mineral phase and quantity (vol %)
- Grain size, exposed surface area and surface deformities
- Alteration and weathering features, such as weathering rims and sulphide oxidation
- Association of different mineral phases
- Spatial distribution of mineral phases in, or adjacent to, areas of weakness, such as fractures and veins
The spatial distribution of different mineral phases relative to areas of weakness will indicate their relative exposure in waste rock after excavation and exposure. Weakness may result from minerals that hydrate (e.g., clay alteration minerals) or dissolve (e.g., gypsum), or physical features such as fractures and veins (Price, 1989).
Users of petrographic analysis should be aware of its limitations. The dimensions of a thin section are relatively small and a large number of sections may be required to accurately characterize heterogeneous materials. Petrographers should note grain size limitations, unidentified phases, any uncertainty in mineral identification, potential losses of material during section preparation and recommendations for alternative techniques. Potentially key mineralogical properties that petrographic analysis cannot distinguish are different carbonate species or the identity of mineral phases whose volume is < 0.2-0.5 vol% or < 50 µm for silicates and < 5-10 µm for sulphide grains. The grain size cutoff prevents mineral identification in fine tailings.
Mineral abundance can be estimated semi-quantitatively from a visual scan or quantitatively from a far more time-consuming point counting. Given the potential limitations in mineral identification with petrographic analysis and the lack of automated procedures, point counting is usually better conducted using SEM/EDX or electron microprobe image analysis.
SEM or Rietveld XRD analysis should be used to confirm results, measure unidentifiable minerals and small grains, and provide more quantitative measurement of mineral abundance. Like most other forms of mineralogical techniques, petrographic analysis is dependent on the skill of the operator. Care should be taken to base mineral identification on the optical evidence and not speculation about the expected composition or theories related to deposit and rock formation.
5.4.9.3 X-Ray Diffraction
X-Ray diffraction identifies mineral phases and measures their quantity from the peaks created by the scattering of radiation by the three dimensional arrays of atoms unique to each minerals. Mineral phases are identified by comparing the locations and intensities of the diffraction peaks with those of mineral reference standards in the International Center for Diffraction Data database. XRD is not limited by grain size and is able to distinguish minerals such as pyrite and marcasite with similar composition but a different crystal structure. XRD has traditionally provided semi-quantitative data.
The two important advantages of Rietveld XRD analysis are the quantitative nature of the data and the low detection limits (Raudsepp and Pani, 2001 and 2003). Rietveld XRD analysis calculates diffraction patterns for each mineral phase from powder XRD data and fits them to the observed powder diffraction pattern. Detection limits for different mineral phases using the Rietveld method may be as low as 0.1 to 0.2 wt%, if there are no overlaps from peaks of other mineral phases (note petrographic estimates of mineral abundance are expressed in vol.%).
The Rietveld method requires that the sample be ground under alcohol to an average particle size of < 5 µm. Alcohol minimizes heat production during grinding, protects the crystal structures of delicate minerals such as micas from damage, and disperses the sample, thereby preventing clumping. A particle size of < 5 µm minimizes micro-absorption and preferred orientation and improves the reproducibility of the diffraction pattern.
Detection limits for mineral abundances depend on:
- XRD instrument, particularly detector sensitivity
- Counting time per point and frequency of analyzed points
- Subjective skill of the operator
- Composition of material, particularly the degree of peak overlap
Potentially important peak overlaps are the main peaks of pyrite and sphalerite, chalcopyrite and calcite, and biotite and illite/muscovite. Other limitations of XRD include an inability to identify the composition of solid solution minerals, fracture coatings, minerals present in trace amounts, and disordered or amorphous minerals such as hydrated sulphates and secondary clay minerals. Phyllosilicate clay mineral species, such as smectite and kaolinite, can be identified by the difference in changes to the interlayer spacing caused by K, Mg, heating and glycol pretreatments. Again, XRD is not a stand alone technique. It needs support of visual and petrographic analysis and occasionally SEM-EDS or electron microprobe.
5.4.9.4 Electron Microprobe
Electron microprobe (EM) accurately measures the elemental composition of selected mineral grains in polished sections, which may be needed to determine the concentration of major or trace constituents.
Electron microprobe may be used to determine the chemical composition of carbonate minerals, especially ankerite and Fe-bearing dolomite, but also other carbonate species, such as siderite, that have a variable composition (solid solution). Where carbonates that are not net neutralizing may be present, microprobe analysis of the chemical composition of selected carbonate minerals is used to measure the proportion that is net neutralizing (Ca and Mg) and not net neutralizing (Fe and Mn) (Frostad et al., 2003).
Measurement of the concentration of trace elements in different mineral phases may be needed to determine the accuracy of assumptions made in interpretation of geochemical results. For example, electron microprobe may be used to measure the proportion of Ba and Pb that occur as acid insoluble sulphate. Measurement of the concentration of trace elements in different mineral phases may also be used to predict conducive conditions for and the relative rate of trace element release, for example, whether Se occurs in sulphide minerals and will be released by oxidative dissolution.
5.4.9.5 Scanning Electron Microscope and Energy Dispersive X-ray Spectrometer
Scanning electron microscopy (SEM) produces a backscattered electron image in which the average atomic number of minerals determines the shade of gray. Silicate minerals with a lower average number appear dark gray, while sulphide minerals with higher atomic numbers are a lighter gray. Portions of the gray-scale can be expanded to differentiate between minerals such as different sulphide minerals with similar average atomic numbers.
Energy dispersive X-ray spectrometry (EDS) measures the elemental composition of small areas of interest and can be used to determine the mineral phase(s) associated with different shades of gray in the SEM image. Major and minor element analysis of polished surfaces by EDS may be semi-quantitative or quantitative.
Used together, SEM/EDS can be used to measure a wide variety of mineral properties:
- Quantification of mineral phases
- Elemental composition
- Grain and particle size distribution and spatial arrangement
- Mineral association
- Number and size of structural deformities and weathering features
Digital image analysis using SEM/EDS software and systems such as quantitative evaluation of minerals by scanning electron microscopy (QEMSCAN®) and mineral liberation analysis (MLA) can provide automated measurements (Lotter et al., 2002; Gu, 2003). Automated SEM/EDS is a more expensive, but also a more comprehensive, alternative to XRD.
5.4.9.6 Other More Specialized Techniques
There are a number of specialized microbeam mineralogical techniques available that measure smaller depths or areas (e.g., surface alteration or coatings), different oxidation states, isotopes, types of bonding, adsorption modes or with lower detection limits than electron microprobe or SEM/EDS. Examples include:
- Laser ablation ICP-MS
- Proton induced X-ray emission (PIXE)
- Secondary ion mass spectrometry (SIMS)
- X-ray absorption spectroscopy or X-ray absorption near edge structure (EXAFS, XANES)
Laser ablation is used for isotope and elemental analysis of thin layers of weathered, precipitated or included material. Day and Sexsmith (2005) used laser ablation to measure the concentration of selenium in reactive minerals at a coal mine experiencing elevated selenium concentrations in the drainage.
5.4.9.7 General Comments
Mineralogical testing is a required, not an optional, analysis. Mineralogical assessment is generally required for a ‘representative’ sub-set of static test samples and each kinetic test sample. Mineralogical data will indicate which minerals likely contributed to test results and the likelihood they will contribute similar amounts in the field. Properties of interest will depend on the mineralogical composition, questions raised by other test work and site-specific weathering conditions.
Careful planning is required to obtain mineralogical information at a reasonable cost. As with other analytical procedures, analysis should occur on the materials and compositional fractions of concern. Some information on mineralogy and mineral distribution may already be available in drill logs, exploration reports, metallurgical test work and academic reports. When requesting mineralogical analysis, it is recommended to provide information on sample geochemistry and any other relevant information (e.g., the type of ore deposit) to the mineralogist/petrographer, as this will help determine the protocol for sample preparation and in the interpretation of results. Generally, the more lines of evidence are available, the more accurate the resultant mineral identification.
Recommended mineralogical methods are as follows:
- Mineral abundance - Rietveld XRD and petrographic analysis – may use image analysis with SEM/EDS instead of XRD
- Mineral spatial distribution - Visual plus petrographic analysis or SEM/EDS
- Mineral chemical composition - Electron microprobe or SEM/EDS
- Mineral physical features - Visual plus petrographic analysis and/or SEM/EDS.
The costs of mineralogical analysis generally are similar to those of ABA and less than the costs of kinetic testing. Potential costs associated with inadequate mineralogical understanding are often prohibitive in terms of consultant fees, environmental risks, and delayed regulatory approval. It is important to recognize that the use of mineralogical information in the selection and design of static and kinetic tests and the interpretation of their results can only occur if the mineralogical analysis is completed prior to these activities.
5.4.10 Net Acid or ARD Potential
Two basic types of test are available for determination of the net acid or acid rock drainage (ARD) potential: acid base accounting (ABA), that measures net acid potential through independent determination of acid generating and neutralizing content, and the net acid generation (NAG) procedure, which generates a single value that can be used to indicate the likelihood of net acid generation. On a global scale, use of ABA and paste pH predominates, with the NAG test commonly used in many regions, particularly Australia, New Zealand and SE Asia.
ABA and NAG tests are relatively inexpensive and can be applied to large numbers of samples. The results from these tests can be used for identification of samples requiring additional testing (e.g., kinetic testing) to more definitively determine acid generation potential (AP). In addition, the tests may provide operational screening criteria for mine waste classification and management. However, some differences exist in the ability of the tests to predict acid generation potential. Acid base accounting should always be conducted, while the NAG test may or may not be included, depending on circumstances (for instance, if there is little or no sulphur present or the ABA results indicate a significant excess of NP, the NAG test provides little additional information).
As described in the prediction section for coal mining, ABA methods were initially developed for the coal mining industry and later adapted for use in metal mining. Although all methods incorporate an independent determination of AP and NP, many different protocols are available and in use. Table 5-1 presents the most common methods and summarizes advantages and limitations associated with each type of test. Results from ABA methods need to be interpreted in context with mineralogical information.
In general, the determination of the AP as part of ABA testing is conducted through analysis of one or more sulphur species. The theoretical relationship between sulphur content and AP is as follows: AP (kg CaCO3/tonne)[1] = 31.25 x S (%).
Sulphur species identified generally include total sulphur and pyritic (or sulphide) sulphur. Other sulphur species frequently determined (either through direct analysis or calculated by difference) include sulphate sulphur, organic (or residual) sulphur, and sulphate associated with barite and alunite. The acid potential can be calculated from total sulphur content (the most conservative approach) or the acid potential can be based on the concentration of one or more sulphur species to provide a more refined estimate of the amount of reactive sulphur present. In the case of coal, it is important to discount the proportion of sulphur associated with organics when determining AP. Similarly, sulphur occurring in the form of non-acid generating sulphate minerals, such as gypsum and barite, should be discounted when information on sulphur speciation is available.
Measurement of AP is often relatively simple and interpretation of results is generally relatively straightforward. However, more interpretation of analytical results is typically needed for tests developed to measure NP because of the widely variable solubilities and reaction rates of potentially neutralizing minerals (e.g., carbonate and silicates), the differences in aggressiveness of the various methods used to determine NP[2], and the different reaction conditions and titration endpoints prescribed for each test. Because the resulting value for the NP is highly sensitive to test protocol and the nature of the NP minerals, it is important that any ABA program makes use of the methodology that is most appropriate for a given objective and application. It is also important that at least one single test method is used throughout the program to ensure that the results are internally consistent. Although perhaps imperfect, the advantage of using “standard” methods for determination of NP, such as the Sobek and modified Sobek methods (see Table 5-1 for description), allows for comparison against a vast body of references values from other sites. The values for AP and NP are combined mathematically to indicate whether a sample has a stoichiometric balance that favours net acidity or net alkalinity.
The net potential ratio (NPR) and net neutralization potential (NNP)[3] are calculated as follows:
NPR = NP/AP and
NNP = NP – AP (kg CaCO3/tonne)
Table 5-4 is an example of ABA results, including summary statistics. Figure 5-6 provides an example comparison of NP calculated from total carbon measurements vs. NP using the modified Sobek method. NP is calculated from total carbon using the following formula, which assumes all carbon in the sample occurs as calcite (CaCO3):
NP (total C) = %C x 83.3
When NP is estimated using surrogate analyses (e.g., from total carbon or calcium), results should be reviewed to ensure that these relationships are applicable to all material types and over the full range of NP values observed.
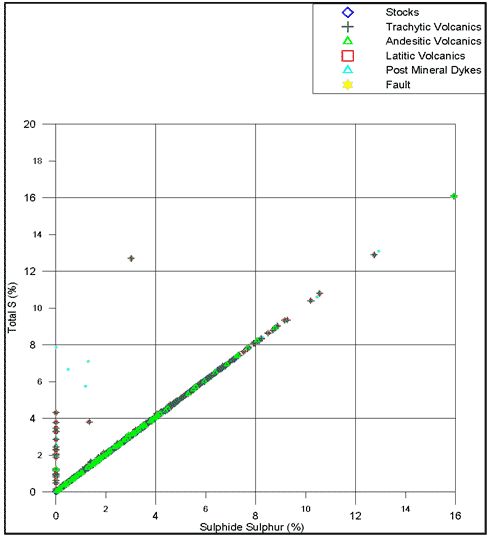
Figure 5-7 compares total sulphur content against sulphide sulphur content. If a quantifiable relationship can be established, then determination of total sulphur may suffice for future purposes. Figures 5-6 and 5-7 are just two of the many graphs that can be used to interpret ABA results.
|
|
Paste pH |
Total |
Sulphate |
Sulphide |
NP |
AP |
NNP |
NPR |
Pit A |
Minimum |
7.6 |
0.01 |
0.005 |
0.01 |
9 |
0.15 |
-189 |
0.2 |
25th percentile |
8.2 |
0.62 |
0.02 |
0.61 |
57 |
19 |
-38 |
0.7 |
|
Median |
8.4 |
2.18 |
0.05 |
2.14 |
81 |
68 |
8 |
1.1 |
|
75th percentile |
8.6 |
3.67 |
0.08 |
3.60 |
98 |
114.5 |
54 |
3.6 |
|
Maximum |
9.5 |
9.35 |
0.18 |
9.26 |
222 |
292 |
201 |
113 |
|
Pit B |
Minimum |
7.4 |
0.002 |
0.005 |
0.002 |
10 |
0.15 |
-471 |
0.1 |
25th percentile |
8.5 |
0.68 |
0.03 |
0.54 |
40 |
21 |
-38 |
0.6 |
|
Median |
8.7 |
1.59 |
0.05 |
1.45 |
56 |
50 |
13 |
1.3 |
|
75th percentile |
8.9 |
3.04 |
0.07 |
2.91 |
85 |
95 |
45 |
3.1 |
|
Maximum |
9.5 |
18.6 |
9.68 |
18.39 |
294 |
581 |
274 |
733 |
The NAG test is used in association with ABA to classify the acid generating potential of a sample. The NAG test involves reaction of a sample with hydrogen peroxide to rapidly oxidize any sulphide minerals. Both acid generation and acid neutralization reactions occur simultaneously and the net result represents a direct measure of the amount of acid generated. A pH after reaction (NAG pH) of less than 4.5 indicates that the sample is net acid generating and the amount of acid is determined by titration and expressed in the same units as ABA.
Several variations of the NAG test have been developed to accommodate the wide geochemical variability of mine waste materials and to address potential interferences. The two main static NAG test procedures currently used are the single addition NAG test and the sequential NAG test. The sequential NAG test may be required for high sulphide sulphur samples to provide a measure of the total acid generating capacity and on samples with high S and high ANC. Specific methodologies are also required for evaluating material with high organic carbon content such as coal rejects and wash plant wastes. Further information on NAG tests and procedures are presented in the AMIRA ARD Test Handbook (AMIRA, 2002).
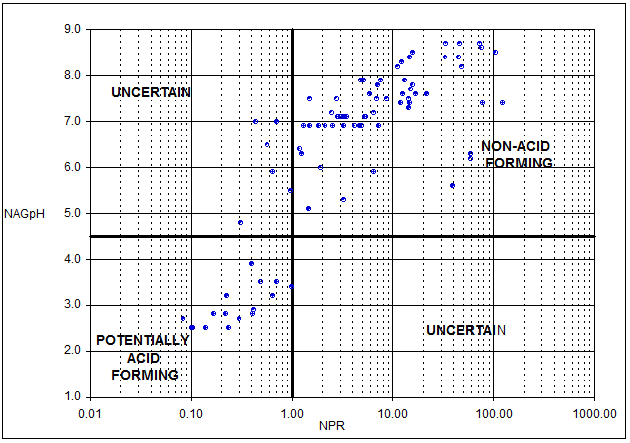
Figure 5-8 shows how ABA and NAG can be used together to improve prediction confidence, identify uncertain samples and better define cut-off criteria for material classification.
Figure 5-8 is a plot of NPR (an ABA parameter) and NAG pH and identifies four quadrants. Samples with NPR greater than 1 and NAG pH greater than 4.5 plot in the non-acid forming quadrant and samples with NPR less than 1 and NAG pH less than 4.5 plot in the potentially acid forming quadrant. Samples with conflicting ABA and NAG results plot in the “uncertain” quadrants. In the sample set shown in Figure 5-8, six samples plot in the upper left hand “uncertain” quadrant and follow up testing can be targeted on these samples to confirm the classification. The results also show that all samples with NPR greater than 1 plot in the non-acid forming quadrant and hence a cutoff NPR of 1 is likely to be appropriate for materials represented by the samples in this data set. This type of analysis can be used to develop site-specific criteria for the identification of acid generating rock types and to define an appropriate factor of safety to minimise the risk of misclassification. For example, for material represented in Figure 5-8, an NPR of 1.5 is likely to provide a high factor of safety for classification of non-acid forming material.
Paste pH is a simple, rapid, and inexpensive screening tool that indicates the presence of readily available NP (generally from carbonate) or stored acidity. The outcome of the test is governed by the surficial properties of the solid material being tested, and more particularly, the extent of soluble minerals, which may provide useful information regarding anticipated mine water quality. For example, acidic paste pH values in combination with elevated sulphate sulphur generally suggest the presence of acidic sulphate salts that could cause short-term or long-term water quality issues.
5.4.11 Short-Term Leach Tests
Although protocols for static (or short-term) leach tests vary widely, all tests measure readily soluble constituents of mine wastes and geologic materials. The short-term nature of static leach tests provides a snapshot in time of a material’s environmental stability. Test results depend entirely on the present disposition of the sample (e.g., unoxidized vs. oxidized; oxidation products absent vs. oxidation products present). For reactive rocks (e.g., material that contains oxidizable sulphur), the transient processes that lead to changes in solution chemistry during water-rock interactions develop over periods of time that are much greater than is stipulated in the testing protocols. Therefore, the results from short-term leach tests generally cannot be applied to develop reaction rates and predict long-term mine water quality, but should instead be used to get an initial indication of parameters of constituents of interest. In addition, metal loadings can be calculated from short-term leach tests, as illustrated in Figure 5-9, where loading rates (in milligrams per kilogram [mg/kg]) are compared against initial sulphate content.
It is important to select the method that most closely simulates the site-specific ambient environment and leaching conditions (e.g., solution to solid ratio, nature of lixiviant, grain size, agitation). In addition, selection of a test method has to take into account the anticipated use of the leach test results (e.g., for prediction of seepage vs. runoff quality, incipient vs. terminal water quality). Regulatory requirements and expectations may also govern selection of a particular methodology. Many jurisdictions have well-defined regulations for evaluation of metal mobility and potential impacts to water resources and in such cases use of a test with regulatory status may be compulsory. In instances where such a test is required but where the mandated protocol has no bearing on site-specific conditions (e.g., the prescribed use of acetic acid in the TCLP test), use of an additional, and more appropriate, alternative short-term leach test is recommended to allow for a more realistic estimate of future mine water quality. Similarly, modifications to standard leach test protocols should be considered to take into account site-specific considerations and improve the tests’ predictive ability.
5.4.12 Laboratory Kinetic Tests
Laboratory kinetic testing methods are used to validate and interpret static test methods, and predict long-term weathering rates and the potential for mine wastes and geologic materials to release discharges that may have impacts on the environment. Both acid generation and metal leaching can be evaluated through kinetic testing.
The results from kinetic testing are frequently used in combination with data from static test, mineralogical analyses and geochemical modeling to evaluate geochemical controls on leachate composition and conduct water quality prediction under a range of conditions. Similarly, kinetic testing results are often scaled up and used in combination with water balances for mine facilities to determine loadings and associated potential impacts to the receiving environment. Depending on the end use of the kinetic test results, results may be expressed in terms of leachate quality (mass released/unit leachate volume), mass-based loadings (mass released/total mass/unit time), or surface-area-based loadings (mass released/total surface area/unit time). For loading calculations, a water balance for the test cell and information on the mass and the surface area of the test charge is required. The results of the laboratory tests then need to be scaled to the mass or surface area of the mine waste. Geochemical reactions and reaction rates most commonly monitored throughout the testing include sulphide oxidation, depletion of neutralization potential, and mineral dissolution.
Kinetic testing procedures are complex, time-consuming, and require operator skill to generate consistent results. For any kinetic test conducted, the objectives and limitations of the method used should be acknowledged before starting the program so that it is clear what information will be delivered from the tests conducted. This will ensure accountability and value for efforts and costs expended.
There is no single test that produces all of the chemical information required to evaluate all mine wastes under all conditions of disposal. In all cases, a sample is subjected to periodic leaching and leachate is collected for analysis, but the various methods available may differ in the amount of sample used, the particle size of the sample, effluent sample volume, test duration, degree of oxygenation, or nature of the lixiviant. Therefore, it is important that the objectives of kinetic testing are clearly defined so that an appropriate test method is selected and adjusted to simulate site-specific conditions and the intended use of the data produced. By the same token, conducting standard humidity cell tests (e.g., using the ASTM protocol – see Table 5-1) is very useful to allow comparison with the significant amount of information on kinetic test results available in the literature. A second phase of kinetic testing may be implemented or field testing may be considered if it is decided that tests representing site-specific conditions are required.
The two laboratory kinetic tests in general use are the humidity cell tests (HCT) and column tests. HCTs represent a standardized test under fully oxygenated conditions with periodic flushing of reaction products. No standards are available for column tests, and column tests can simulate different degrees of saturation, including flooded and oxygen-deficient conditions. Column tests are typically larger scale than humidity cell tests. Figure 5-10 is a photo of a typical HCT setup.
HCTs are primarily intended to generate information on weathering rates of primary minerals (e.g., sulphides); information that can be used to estimate the potential for future net-acid conditions. Dissolution rates of readily soluble primary and secondary minerals present at the onset of testing (e.g., gypsum, hydrothermal jarosites) can also be derived from HCT results. In combination with geochemical modeling, HCT leachate results can be, and are frequently, used to make inferences with respect to drainage chemistry, but due to a lack of equilibration with primary and secondary minerals during HCT operation, such an evaluation has to be conducted with caution.
Column tests differ from HCT by having a design that allows contaminants released from primary minerals to precipitate at their natural rates as secondary minerals (Price, 2009). By providing information on the combined effects of primary and secondary minerals, columns provide a more accurate measure of drainage chemistry. Column tests may be modified to simulate the effects of site-specific climate conditions and mitigation measures such as covers and amended mine wastes. Transfer of oxygen, which is not limiting in HCTs but may be in columns, must be understood in column testing. Figure 5-11 is an example of pH and concentration trends and presentation of results from a column or humidity cell test.
For both HCT and column tests, it is imperative that the test charges be characterized before kinetic testing begins and after kinetic testing has been completed. The information on the test charges may provide important constraints to assist in the interpretation of test results, and may also provide information that can be used for quality control purposes by comparing measured mass removal against calculated mass removal from the leachates.
The required duration of kinetic testing is an area of controversy. The duration of the test depends on the characteristics of the sample and test objective. Although a minimum length of 20 weeks is sometimes referenced, there is little technical basis for the 20-week recommendation. If the objective is to determine whether a sample will generate acid, kinetic tests should be conducted until acidic drainage is produced or until depletion calculations can be used reliably to predict acid generation potential. Another common endpoint for the kinetic testing is when leachate parameters are relatively constant with time.
5.4.13 Field Methods
Field methods to determine acid generation and metal leaching potential range from rapid very small-scale tests to monitoring of full-size mine facilities for extended periods of time. In all cases, the advantage of the field methods is that on-site materials are used and an added benefit is that that most field tests allow for evaluation of weathering reactions under ambient conditions, including seasonal effects and discrete events such as intense storms or snowmelt. The greater the amount of material included in the test, the greater the likelihood that a well-designed method will adequately reflect the chemical and mineralogical composition and physical properties of a mine facility. The larger amount of material will better represent particle size distribution, porosity, hydraulic conductivity, gas ingress, and transport. Disadvantages of field cells are related to the time required to generate reliable field reaction rates, challenges with comprehensive geochemical characterization of the large test charges, problems (especially prior to mining) related to obtaining large sample volumes, and the space needed to test a large number of different material types.
The simplest “field” test is the 5-minute field leaching test (FLT) recently developed by the USGS to simulate the chemical reactions that occur when geological materials are leached by water (Hageman, 2007). The test is considered by the USGS a useful screening procedure that can be used as a surrogate for laboratory leach tests such as the Synthetic Precipitation Leaching Procedure (SPLP), (see Table 5-1).
Wall washing allows for evaluation of runoff quality from an isolated section of in situ rock face after application of a controlled amount of irrigation (Figure 5-12). This wall washing test is considered to represent a very useful order-of-magnitude estimate of contributions from exposed open pit walls or underground mine faces.
Pilot cells (Figure 5-13), test piles, test plots (Figure 5-14), or test pads are constructed for long-term monitoring of relatively large quantities of material. Large-scale field columns (field lysimeters), to be operated under natural precipitation conditions, can also be useful.
Monitoring can be conducted under ambient field conditions, or under controlled conditions, using artificial irrigation. The larger scale relative to laboratory tests results in field test plots having more representative sample dimensions and particle size, in the case of waste rock, and minimizes impacts from boundary effects, sample heterogeneity, and reduced grain size. A comprehensive characterization of the test charge is required. In combination with a good understanding of the water balance for the test pad (achievable through meteorological monitoring or controlled application of infiltration, or both), reaction rates and loadings can be developed for extrapolation to full-scale mine facilities. Longer monitoring durations may be required because of lower field temperatures, intermittent drying, and lower reactivity of field cell test charges relative to the finer-grained materials commonly included in laboratory tests. It may be advantageous to operate field tests during the complete life of mine to identify potential long-term releases.
On-site monitoring of historical and newly-constructed mine facilities (e.g., waste rock pile, tailings impoundment, pit wall and adits) can provide very useful information regarding weathering rates and discharge quality under ambient conditions. By definition, monitoring results of this nature are representative of the facility and existing conditions as a whole, but prediction of future conditions may be hindered by the sluggish rate of reaction relative to smaller scale tests. Also, a comprehensive understanding of chemical and physical material characteristics is not generally feasible, nor is a comprehensive understanding of the water balance, water movement and the role of atmospheric gases. This may limit the interpretive value of direct monitoring of mine facilities for the prediction of future water quality and potential impacts to receptors.
5.4.14 Data Management
Proper data management is critical to any geochemical characterization and mine water quality prediction effort, and setup and maintenance of a database is an integral component of such a program (Bellefontaine and Price, 2006; Wolkersdorfer, 2008). The primary requirements for a useful and reliable database are that it should be in electronic format, it should be implemented from the beginning of the study, and it should be maintained and augmented throughout all phases of a mining project.
A database should be managed from a central location, with routine backups. The data should be presented in a format that is readily accessible, and appropriate safeguards should be in place to maintain the integrity of the information stored in the database and prevent unauthorized use. Although most databases are designed to store numeric information, increasing use of geospatial data is incorporated by use of geographic information system GIS). GIS provides a means for integrating and interpreting geochemical data within a geospatial context for land use, climate, topography, or ecosystem. The primary function of a database for geochemical data is to act as a comprehensive data repository that can be used to check and maintain data integrity (see Section 5.4.15 on QA/QC), support data manipulation and data interpretation (including mine planning and material scheduling programs), support and guide water quality and other monitoring programs, enable evaluation of compliance with regulatory requirements, and allow for evaluation of historical trends and prediction of future conditions.
One type of database unique to mining is the so-called block model, which is a 3-dimensional computerized representation of the quantity and characteristics of the pit walls, ore, and waste rock. Historically, block models have been resource focused, and have included information on ore grade, lithology, alteration types, principal minerals, fracture density and orientation, and rock competency, all of which are aimed at optimizing resource recovery. To this end, data from exploration drill holes are subjected to a variety of geostatistical analysis methods, such as kriging to quantify the 3-dimensional distribution of ore throughout the mine. However, increasingly, the same block models and geostatistical techniques are also used for environmental purposes, such as development of waste rock management plans and mine water quality prediction. Results of geochemical characterization programs are incorporated in block models, including inputs such as sulphur and sulphide content, NP, paste pH, NAG pH, NCV, carbon, and carbonate content. The combination of resource and environmental parameters in block models allows for prediction of environmental behaviour of mined materials in time and space and identification of requirements for mitigation actions in time and space. Environmental block models should be developed when a 3-dimensional understanding of ARD potential is required, and should then be maintained and refined throughout the life of mine through the ongoing acquisition of additional data. Examples of use of block models are presented in Figures 5-15 and 5-16. Figure 5-15 shows the ARD potential of a highwall remaining exposed after pit lake formation. Figure 5-16 shows the ARD potential of pit walls at the cessation of mining. In both cases, a block model incorporating ABA parameters formed the basis for the evaluations.
5.4.15 Quality Assurance/Quality Control
A rigorous QA/QC program is needed to ensure that geochemical data are reliable and defensible, and that such data can be used for their intended purpose, such as defining the geochemical types and distribution of mine wastes, developing waste management plans, and for mine water quality prediction.
QC is defined as the application of good laboratory practices, good measurement practices, and standard procedures for sampling. QC is also defined as sample preparation and analysis with control points within the sample flow to prevent the reporting of erroneous results. The sampling should include specifications for chain of custody procedures and documentation, sample holding time verification, drying, comminution, storage and preservation, sample labeling, and use of proper sample containers. Physical and chemical tests conducted using appropriate methods and accredited laboratories should produce analytical results with sufficient accuracy and precision for their intended usages. Analytical methods and their repeatability, reproducibility, quantification, and detection limits should meet anticipated requirements (e.g., for classification of geochemical rock types or comparison against water quality standards). Replicate samples, standards, certified reference materials, and blanks should be routinely submitted to ensure and confirm that the analytical results are of acceptable quality. QA is the process of monitoring for adherence to quality control protocols. The DQO of a quality assurance project plan (QAPP) are as follows: accuracy, precision, bias, representativeness, completeness, and comparability. A QAPP will ensure that the proper procedures are established before initiating sample collection and analysis, and that procedures are maintained throughout all stages of a geochemical program. In addition, corrective actions are prescribed through a QAPP. A defensible QA/QC program will add costs to an ARD study, but it will also allow timely correction of errors, saving time and money, and enhance the confidence of operators, regulatory agencies, and other reviewers in assessing the data. A QAPP will help balance the costs of implementing a quality-assured program against the potential liabilities associated with a poorly-designed and executed geochemical characterization program.
The data validation and assessment protocols for geochemical data generated in support of prediction of ARD and metal leaching potential are similar to those used in any type of study that relies on use of analytical results, and the data validation and assessment protocols include a variety of statistical analyses and graphical tools. Geochemical modeling can be useful (e.g., through calculation of the ion balance), while cross checking using results from different types of testing also may provide insight in data quality (e.g., calcium content vs. NP, sulphur content vs. mineralogical composition, measured vs. calculated TDS, NP titration vs. TIC).
5.4.16 Screening and Evaluation Criteria
Screening and evaluation criteria are used to assess whether results from geochemical characterization studies represent a potential impact or risk to a receiving environment at a mine site and to segregate problematic wastes. These criteria can be based on professional and empirical experience, guidance documents, and regulations promulgated for the express purpose of protecting the environment.
Screening and evaluation criteria are commonly used at mine sites for water and mine waste management. Mine waste management involves identification of potentially net acidic or ARD generating (PAG) and non potentially net acidic or ARD generating (NPAG) waste. PAG material is either acidic or predicted to become net acidic in the future. A material will become net acidic if the rate of acid neutralization is unable to keep pace with the rate of acid generation. This inability to maintain neutral conditions may be due to a decrease in the rate of acid neutralization or an increase in the rate of acid generation, or both. NPAG material is predicted to generate near-neutral or alkaline drainage in the future. Materials will be net neutral or alkaline if the rate of acid neutralization keeps pace with the generation of acid (Price, 2009).
Site-specific operational parameters and threshold values are established for waste classification (i.e., PAG vs. NPAG) based on regulatory requirements, literature, and the geochemical test program. Examples of commonly used operational parameters for waste rock management include the sulphur content (including total and sulphide sulphur), paste pH, NNP, net potential ratio (NPR), NCV, NAG test value, or NAG pH and metal content.
Theoretical relationships, empirical data, and evaluation of analytical and logistical constraints should be used to establish screening or evaluation criteria. For example, if a quantitative relationship can be reliably established between ARD potential and sulphur content, a sulphur cutoff can be determined to segregate between PAG and non-PAG waste rock. Similarly, if a relationship between metal leachability and metal content is identified, a metal concentration cutoff can be established to discriminate between material that will or will not affect receiving water quality. Sometimes a combination of methods is needed to classify problematic material, such as paste, pH, sulphur, and NPR.
Guidance documents are available that provide screening criteria for evaluating geochemical test results, in particular those tests related to prediction of ARD potential: ABA (Price, 2009) and NAG test (AMIRA, 2002). These criteria are generally related to specific values for NNP, NPR, NAG pH, and NCV, and can be used to classify mine wastes and geologic materials in terms of their ARD potential. Special care is required when dealing with mining wastes that exhibit both low sulphur contents and low NP because small changes in analytical results can dramatically affect the calculated NPR and the mine waste classification. Therefore, the screening process should be supported by data from a number of analyses and tests, including the mineralogical composition.
5.4.16.1 Acid Base Accounting Screening Criteria for the Net Acid Potential
An acid pH increases the solubility of most metals (Stumm and Morgan, 1996.) and below pH 3.5, the increased dissolved Fe(III) concentration greatly increases the rate of sulphide oxidation (Williamson et al., 2006). Consequently, criteria used to identify materials with the potential for acidic drainage are a key component of sound environmental and fiscal management. The objective is to be both accurate and cost-effective. Criteria may provide useful short cuts and enable cost-effective prediction, but users always need to evaluate the underlying assumptions and limitations and whether the proposed criteria are compatible with the site-specific conditions.
The following criteria are based on practical and theoretical (scientific) considerations, but it should be noted that a different set of criteria may result from site-specific considerations. A more detailed description is provided in Price (2010).
Under near-neutral or alkaline, oxidized conditions, sulphide oxidation (Reaction 1) and dissolution of acidic sulphate minerals (Reaction 2) may produce acid. If not neutralized (Reaction 3), the acid will lower the pH.
Sulphide (pyrite) oxidation: FeS2 + O2 + H2O → Fe(OH)3 + 2SO42- + 4H+ (1)
Acid sulphate (melanterite) dissolution: FeSO4•7H2O + O2 → Fe(OH)3 + SO42- + H2O + 2H+ (2)
Acid neutralization by calcite: CaCO3 + H+ → Ca2+ + HCO3 (3)
The most cost-effective means of predicting whether sulphidic geologic materials are PAG is based on the results of ABA, a series of compositional analyses (static tests) and calculations used to estimate the potential for a near-neutral or alkaline sample to produce acidic drainage if it is exposed to oxygen and water. Acid base accounting consists of:
- Analysis of pH (paste, soil, or rinse pH)
- Analysis of acid generating sulphur species and calculation of acid potential (AP)[1]
- Analysis of neutralization potential (NP)
- Calculation of NP/AP (NPR) and NP-AP (NNP)
The pH analysis measures the chemical effect of particle surfaces on drainage pH and indicates if a sample is already able to produce acidic drainage.
The future potential for sulphidic geologic materials with a near-neutral or alkaline pH to produce acidic drainage if exposed to oxygen and water depends on the relative concentration and reaction rates of acid generating sulphur minerals (AP) and neutralizing minerals (NP). The relative magnitude of the NP and AP is indicated by the NP/AP or NPR. AP and NP are reported as kg CaCO3 equivalents/tonne so they can be compared. A factor of 31.25 is used to convert % S to kg CaCO3 equivalents/tonne based on the assumption that 1 mole of sulphur produces 2 moles of H+ (Reaction 1 and 2) and 1 mole of calcite (CaCO3) neutralizes 2H+ (Reaction 3) as follows:
AP = 31.25 (% sulphide-S + % acid sulphate-S)
Acid neutralization by calcite: CaCO3 + H+ → Ca2+ + H2CO3 (4)
Acid neutralization by calcite: CaCO3 + H+ → Ca2+ + HCO3- (5)
There are two neutralization reactions for calcite. Reaction 4 predominates below pH 6.3. Reaction 5, which requires twice as much NP to neutralize each mole of H+, predominates at higher pH. Reaction 4 is assumed in the calculation of AP (%S x 31.25). With reaction 4, an NPR < 1 is required to produce ARD. With reaction 5, an NPR > 2 is required to prevent ARD. Under near-neutral pH conditions, micro-sites with both reaction 4 and 5 are likely to occur. Consequently, the NPR required to generate ARD will be between 1 and 2. This is why the ratio of NP depletion (moles Ca + Mg) to AP depletion (moles sulphate) measured in humidity cells is typically between 1 and 2 (Figure 5-17).
versus time in weeks (x-axis) for two humidity cells (from Price, 2010)

Assuming that the measurements of AP and NP are correct, samples are (Figure 5-18):
- Potentially net acid generating (PAG) if NP/AP < 1
- Not potentially net acid generating (non-PAG) if NP/AP > 2
- Uncertain if NP/AP is between 1 and 2
Safety factors may need to be added to these criteria to address limitations in the precision or accuracy in sampling, material handling or prediction of the NP and AP. There are many opportunities for over or under estimating the AP and NP (Price, 2009). For instance, preferential deposition of heavier sulphide minerals may result in a tailings beach having a higher AP than the tailings leaving the processing plant. The exposed AP of waste rock may be higher than predicted by analysis of pre-mine drill core or pre-blast hole chips, if sulphides preferentially report to waste rock fines (Table 5-5). Rock types differ in their surface area and therefore their relative contribution to the overall waste rock composition. If PAG waste rock is highly sericitic, it “opens up” like a book, exposing all its AP. In contrast, non-PAG waste rock with most of the NP may be very hard, with relatively little reactive surface area. The net result is a much lower effective NP/AP ratio than predicted by the relative masses of the two rock types and, consequently, a much greater likelihood for generation of ARD.
Table 5-5: AP and NP of > 2 mm and < 2 mm waste rock particle size fractions (from Price, 2010)
| > 2 mm | < 2 mm | < 2 / > 2 | |
| AP (kg CaCO3/t) | 86 | 257 | 3.0 |
| NP-Sobek (kg CaCO3/t) | 32 | 44 | 1.4 |
Oxidation of thiosalts from mineral processing may acidify a tailings water cover (Reaction 6). Oxidation of ammonium (NH4+) from blasting powder, fertilizer and cyanide decomposition may also acidify a tailings water cover (Reaction 7 and Figure 5-19). An initial decline in seepage pH may result from the exchange of cations in the neutral mine drainage for H+ in acidic organic soils below a waste rock dump (Reaction 8 and Figure 5-20).
S2O32- + 2O2 + H2O → 2SO42- + 2H+ (6)
NH4+ + 2O2 → NO3- + 2H+ + H2O (7)
2CH3COOH + SO42- + Ca2+ → 2CH3COO-Ca + SO42- + 2H+ (8)
drainage for H+ in acidic organic soils below a waste rock dump (from Price, 2010)

Other sources of acid in addition to sulphide and acidic sulphate minerals include naturally acidic groundwater and runoff from surrounding areas of sulphide mineralization (Price, 2005a).
The criteria for acid generation potential based on the NPR can be summarized as follows:
Criterion: Sample is PAG if NPR < 1. This criterion is true if there are no “errors” in the estimation of effective NP and AP. Possible errors include:
- Acid generated from AP is neutralized by alternative sources in addition to the NP
- At a very low rate of sulphide oxidation, the neutralization capacity of silicates may be underestimated by NP analyses because their reaction is too slow to be completely measured during a relatively short period of acid digestion
- Sulphur minerals containing the sulphur used to calculate the AP may generate < 2 moles of acid per mole of sulphur
- NP and AP measurements are made on whole samples (e.g., drill chips) of material in which NP is preferentially exposed on surfaces, while AP is unavailable within coarse particles
Criterion: Sample is Non-PAG if NPR > 2. This criterion is true if there are no “errors” in the estimation of effective NP and AP. Possible errors include:
- NP is depleted by acid produced in processes other than by acidic sulphate dissolution or sulphide oxidation, which in well-flushed humidity cells can include NP dissolution by the excess water
- NP produces less acid neutralization than calcite or is incapable of maintaining a near-neutral pH
- Sulphide or acid sulphate minerals may generate or release more than 2 moles of acid per mole of sulphur
- NP and AP measurements are made on whole samples (e.g., drill chips) of material in which AP is preferentially exposed on surfaces, while NP is unavailable within coarse particles
Criterion: 1 ≤ NPR ≤ 2. Assuming no errors in the prediction of the effective AP and NP, the maximum NPR capable of generating ARD will be between 1 and 2. The classification of a sample with an NPR between 1 and 2 may remain “uncertain” until the NPR criterion is refined. The ‘minimum’ sulphur content capable of causing ARD depends on the type of sulphur and the magnitude of the NP. Mined rock often has an extremely low NP. For instance, at the East Kemptville Mine in Nova Scotia, humidity cell samples with 0.07 to 0.19% sulphide-S, NPR of 1 to 2 and NNP > 0 produced acidic drainage (Morin and Hutt, 2006). Great care is required when working with materials containing low AP and NP levels because minor variations can significantly alter the predicted and resulting drainage chemistry. A sulphur cut-off should not be used to assess the ARD potential unless the minimum NP value is known. Even low levels of sulphide can produce ARD if the NP is insufficient to neutralize the resulting acid.
The magnitudes of NP combined with humidity cell measurement of NP removal rates provide rough estimates of the time to NP depletion. NP depletion of 2.5 to 5 kg CaCO3/tonne/year suggested it would take 36 to 72 years to deplete an NP of 180 kg CaCO3/tonne in the backfilled tailings sand in the Snip Mine (Price, 2005b). To support calculations of NP depletion and lag times to acid generation derived from laboratory testing, it is important to set up field test pads as soon as practicable to monitor weathering under field conditions in various geologic materials at the site (Price, 2009).
Observations such as “If this rock was potentially ARD generating, we would have already seen ARD in the dumps, some of which are over 50 years old.” are frequently encountered. However, an absence of ARD after extended periods does not prove it will not occur in the future because depletion of NP may take 10s to 100s of years. For example, it took more than 15 years before acidic drainage was observed at Island Copper, where waste rock contained only a moderate amount of NP (Figure 5-21, Morin and Hutt, 1997).
Other considerations regarding ABA criteria are as follows:
- Calculation of AP, NP and NPR typically assumes oxidizing conditions.
- The question is not whether a material generates acid, because everything generates some acid, but whether it will become net acid due to insufficient NP to neutralize the acid.
- The ARD potential of materials with an NPR between 1 and 2 will depend on the fate of alkalinity (HCO3-) produced by the pH > 6.3 neutralization reaction (Reaction 5).
- NNP = NP-AP is additive rather than a ratio, and can therefore not distinguish between materials with an NPR > 2 and an NPR 1 to 2. Use of the NNP is not recommended for characterizing the future ARD potential (Figure 5-22).
- Drainage chemistry prediction should still be conducted if the NPR > 2 because contaminant concentrations at near-neutral or alkaline pH may yet be above environmental guidelines (Stantec, 2004).
In summary, ABA criteria used to classify materials should be based on practical and theoretical (scientific) considerations. Criteria may provide short cuts, but one always needs to check whether the underlying assumptions or limitations apply to a specific situation. Mineralogical, elemental and humidity cell data are required to check assumptions about chemical species contributing to the ABA parameters and calculation results.
Numerical ABA criteria provided in guidance documents are sometimes misunderstood, used inappropriately and inaccurately described (e.g., the description of guidelines from Price [1997] in Maest et al. [2005]). Always consider the specific situations to which the criteria apply and the details concerning their use.
It is important to recognize that generic ABA criteria cannot substitute for an understanding of the natural environment, the project, the geological materials and the requirements for protection of human health and the environment. Therefore, development of site-specific criteria is necessary based on measurable parameters and a well-informed assessment of the limitations of the results. Practitioners need to decide what information is required to make an assessment, under what conditions ‘short cuts’ are permitted, and when conditions deviate from the ‘expected’. Sensitivity analyses and risk assessment are required to determine the quality and adequacy of the available information.
5.4.16.2 Net Acid Generation Screening Criteria for the Net Acid Potential
Figure 5-23 is the Australian AMIRA (2002) decision tree for determining acid generation potential. Through use of a combination of results from NAG testing, partial ABA testing, and professional judgment, samples are categorized into a number of classes with a range of ARD potentials.
5.4.16.3 Other Screening Criteria
No specific NPR value is regulated in the European Union (EU); rather, site-specific values are developed. At some Australasian sites, an NPR value of 3 is conservatively assumed to be the threshold between potential acid generating and nonacid generating mine waste. However, use of a lower ratio is acceptable only if it can be demonstrated, based on site-specific information, that such a value is sufficiently protective. As with all screening criteria, the burden in on the proponent to demonstrate that these criteria are appropriate and defensible based on site-specific considerations.
Worldwide regulatory jurisdictions have adapted criteria for ARD potential, and some have been promulgated into law. When such criteria exist, their application is generally mandatory, unless use of appropriate and defensible site-specific criteria is allowed under the law. The selected criteria can vary and an understanding of applicable regulations is needed when evaluating results from ABA and NAG tests for the purpose of prediction of ARD potential and identification of mine waste management requirements. Examples of such regulated criteria include an NPR threshold of 3 for nonacid generating waste in New Mexico, an NPR threshold of 1.2 in Nevada, (i.e., 20% excess base), and a three-pronged approach in Quebec based on sulphide content, NNP, and NPR. In Quebec, acid generating material is characterized by sulphide content greater than 0.3%, and, in the absence of confirmatory kinetic testing results, an NNP less than 20 kg CaCO3/tonne or an NPR less than 3. Figure 5-24 is an example plot of ABA results in which a number of screening criteria have been included, delineating the boundaries between materials with a different potential for ARD.
Regulatory criteria also exist for interpretation of results from certain leach tests specifically designed for classification of waste materials and compliance with water quality standards, as indicated in Table 5-1 and on Figure 5-17 (AMIRA, 2002). Examples of such tests include the TCLP, meteoric water mobility procedure (MWMP), and WET tests in the United States, the CEN-series tests in Europe, the Chinese GB tests, and the Brazilian Norma Brasileira Registrada (NBR) tests.
In general, kinetic test results need to be interpreted in the context of all available geochemical information. The following evaluation steps may be of assistance in the assessment of kinetic test results:
- Temporal trends of acidity, alkalinity, sulphate, and pH used to assess rates of acid production and consumption
- Ratio of acid production (using sulphate) vs. acid consumption (using calcium, magnesium, alkalinity) to assess relative rates
- Comparison between observed sulphate generation rate and literature values (Morin, 1997)
- Comparison between observed metal concentrations and water quality objectives (A direct comparison generally should only be used as a screening tool, and should take into account the differences in solid to liquid ratio between the test and the ambient environment.)
- Comparison between kinetic test results and findings from ABA, NAG test, mineralogy, static leach testing, and field water quality
- Comparison between kinetic test results and water quality from analog sites (i.e., geo-environmental approach)
- Geochemical modeling to identify controls on leachate composition
- Development of relationships between sulphate concentrations and those of constituents of interest that can be extrapolated to field conditions through sulphide oxidation modeling or calibrated against field measurements of sulphide oxidation
In the absence of regulatory criteria, and frequently in addition to regulatory criteria, site-specific screening criteria should be developed. These criteria should be based on a thorough geochemical characterization of the material at hand. Results from ABA, NAG testing, mineralogical examination, leach testing, and kinetic testing are used to develop an internally consistent understanding of acid generating potential, culminating in identification of a small number of criteria (generally one or two) that can be used to reliably classify mining wastes and geologic materials according to their ARD potential. To be of value in an operational setting, these criteria need to be based on parameters that can be rapidly determined onsite with a high degree of confidence. Visual methods (e.g., rock type, alteration type, pyrite content) and laboratory determination of total sulphur (Leco), sulphide sulphur (Leco minus weak acid soluble-S), Sobek NP, total carbon (Leco), inorganic carbon (HCl soluble), NCV, and NAG pH are the most commonly used operational waste management tools.
Although development of screening criteria is commonly aimed at identifying the net acid generation potential of a mine waste or geologic material, the process of evaluation of potential environmental impacts should not stop there. The material classified as non net-acid generating should still be assessed for drainage quality. NMD and SD from non net-acid generating material may continue to be a cause for concern even in the case of waste management strategies that include, for example, segregation of PAG from NPAG waste rock or encapsulation of PAG rock by NPAG rock.
5.4.17 Reporting
Reporting is an integral part of an ARD-related study. In addition to including tabulations of analytical results, reported information needs to be presented in a format that provides proper interpretation. This requires calculation of descriptive statistics and use of a variety of graphical representations developed for evaluation of results from ABA, NAG testing, and kinetic testing. Price (2009) or Wolkersdorfer (2008) provide a comprehensive overview of the most commonly used table templates, calculation sheets, and graphs.
These procedures must be documented and submitted as part of the report because the reviewer of an ARD study may not be familiar with all analytical and sampling procedures. Also important is a discussion of QA/QC aspects and their bearing on data reliability and defensibility.
At a minimum, the report needs to contain all predictions of environmental behaviour, including the approach and tools used (e.g., geochemical modeling code, statistical software), assumptions incorporated in the predictions, the prediction results, and a discussion of uncertainties and limitations associated with the predictions. Frequently, a report will also include recommendations for further activities related to data collection or evaluation, an interpretation of results in terms of potential environmental impacts, and an assessment of measures that can be used to prevent, minimize, or mitigate such potential effects.
- ↑ The acid potential is also referred to as the maximum potential acidity (MPA), expressed in the units of kg H2SO4/t and calculated as follows: MPA (kg H2SO4/t) = 30.6 x S(%)
Previous Page (Page 1) of Chapter 5